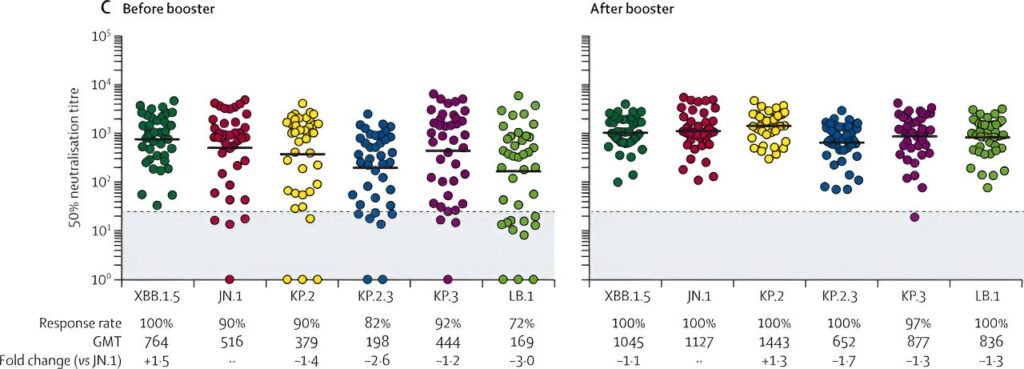
Humoral immune response after mRNA Omicron JN.1 vaccination. Credit: Lancet Infectious Diseases (2024). DOI: 10.1016/S1473-3099(24)00603-0
The autumn wave of the new coronavirus is sweeping across Germany. Affected people primarily suffer from coughs, colds, sore throats, and fevers, but also headaches, pain in the limbs, general weakness, and shortness of breath. New variants and subtypes of SARS-CoV-2 are constantly emerging, so like influenza viruses, coronavirus vaccines must constantly adapt.
Currently, the virus variant Omicron JN.1 and its sublineages KP.2 and KP.3 are predominant. Since August this year, a BioNTech/Pfizer vaccine named Comirnaty omicron JN.1, adapted to the current variant, has been on sale in Germany. Research by the Department of Rheumatology and Immunology at the Medical University of Hannover (MHH) has scientifically proven the effectiveness of the new Omicron Booster.
The researchers, in collaboration with the German Primate Center in Göttingen, published the first paper on the subject in the journal Lancet Infectious Diseases.
Spike protein blueprint
The new booster is another mRNA vaccine, so it is made up of messenger RNA. Principle: mRNA contains the genetic information for the blueprint for the so-called spike protein, which is present on the surface of the coronavirus and helps the virus enter cells. The immune system recognizes the protein as foreign, triggers a protective response, and develops immune defenses.
Comirnaty omicron JN.1 is a so-called monoclonal vaccine. That is, it only contains mRNA from this omicron variant. At the end of June, the European Medicines Agency (EMA) recommended marketing authorization for a new coronavirus disease (COVID-19) mRNA vaccine based on the JN.1 spike protein.
“However, data on human immune responses and real evidence of protection from vaccines were still withheld until our study,” said the senior internist, who is leading the immunology research with fellow professors at the clinic. Professor Alexandra Doepfer-Jablonka says: Dr. Georg Behrens.
Protection against serious disease progression
To obtain such data, researchers studied 42 MHH employees who received the new vaccine as part of the COVID-19 Contact (CoCo) Study, which has been ongoing since the coronavirus pandemic began. The immune response was measured. As a result, antibodies against the current Oomicron variant increased significantly approximately two weeks after the new booster vaccination.
Prof. Behrens pointed out that the participants already had high immunity against various SARS-CoV-2 variants due to previous vaccinations and surviving corona infections, adding: “However, we believe that Omicron JN “We assume that the new mRNA vaccines against .1 will be effective,” he added. You can protect yourself from hospitalization and post-COVID-19 sequelae caused by the latest virus variants. ”
According to the Standing Committee on Immunization (Stiko), booster vaccination with newly adapted vaccines is recommended for people aged 60 and over. In addition, all people who are at particularly high risk of severe disease progression due to underlying medical conditions should receive a booster immunization. Stiko also recommends booster vaccinations for nursing home residents and workers in the long-term care and health care sectors. Booster vaccinations, like influenza vaccinations, must be repeated annually.
Vaccine makers BioNTech/Pfizer and Moderna are already working on a combination vaccine that protects against the coronavirus and influenza virus at the same time.
Further information: Christine Happle et al, Humoral immunity after mRNA SARS-CoV-2 omicron JN.1 vaccination, The Lancet infection (2024). DOI: 10.1016/S1473-3099(24)00603-0
Provided by Hannover Medical University
Citation: Study confirms effectiveness of new Omicron Booster (September 26, 2024) Retrieved September 26, 2024 from https://medicalxpress.com/news/2024-09-effectiveness-omicron-booster.html
This document is subject to copyright. No part may be reproduced without written permission, except in fair dealing for personal study or research purposes. Content is provided for informational purposes only.