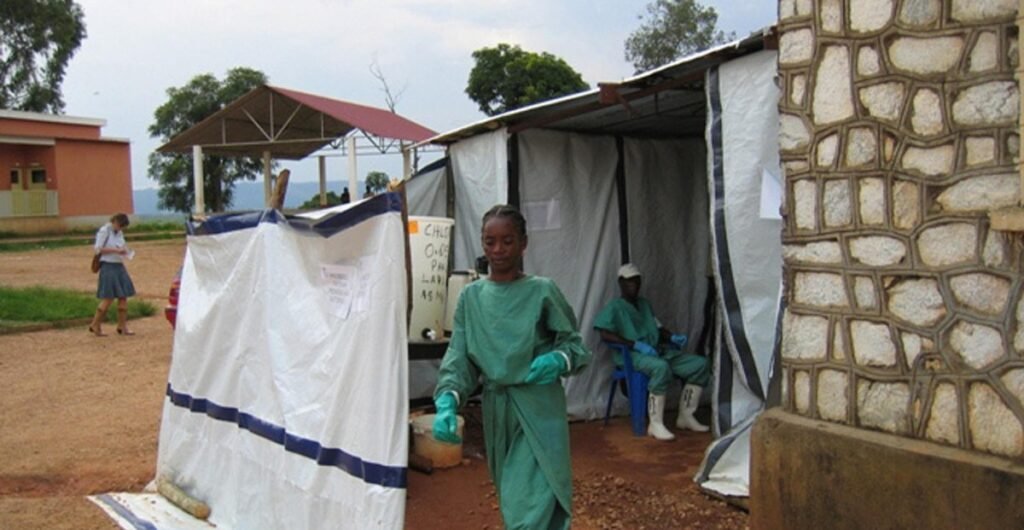
An outbreak of Marburg disease, a serious and often fatal virus closely related to Ebola, is spreading in Rwanda. As Africa struggles with increasingly complex public health challenges, this time offers a rare ray of hope: a chance to test new vaccines that could potentially save lives across Africa in the future.
The World Health Organization (WHO) announced in a press conference on Thursday that the country’s first patient infected with the virus died on September 8th. As of September 29, a total of 36 cases and 11 deaths have been reported, making it the largest Marburg disease outbreak in history. Most of the infections are among healthcare workers at two hospitals in the East African capital, Kigali, but cases have been reported in seven of Rwanda’s 30 districts. This is the first time this virus has been confirmed to cause an infectious disease in Rwanda.
The WHO suggested that while the outbreak was likely to spread to neighboring countries, the risk of further global spread was low. Humans usually become infected when they come into contact with the Egyptian fruit bat, which carries Marburg disease and lives in parts of Africa, the Middle East, and western Asia. However, once a person is infected with the virus, they can spread it to others through bodily fluids or contact with contaminated surfaces or materials (such as clothing or bedding). This mode of transmission means that Marburg virus does not spread as easily as, for example, COVID-19 or other respiratory diseases.
Sign up here to explore the big, complex problems facing the world and the most efficient ways to solve them. Sent twice a week.
However, as has been seen with previous Marburg virus outbreaks, travelers returning from countries with outbreaks can bring the disease back to their home countries. One person who came into contact with an infected person in Rwanda has already traveled to Belgium, highlighting the possibility of isolated cases occurring overseas.
Historically, Marburg virus disease has been fatal in approximately 50 percent of cases, but several previous infections have had mortality rates of over 80 percent. People experience high fevers, severe headaches, extreme fatigue, rashes, bloody diarrhea, abdominal pain, unexplained bruising, and bleeding from the nose and gums. Symptoms may appear 2 to 21 days after initial infection.
All previous Marburg virus outbreaks have occurred in sub-Saharan Africa, with eight cases and five deaths recently confirmed in Tanzania, and 16 cases and 12 deaths in Equatorial Guinea. did. Both outbreaks occurred in 2023. From 1967 to 2008, Marburg disease was reported in the United States, Germany, Yugoslavia, and the Netherlands among travelers returning from Uganda.
To date, there are no approved treatments or vaccines for Marburg virus disease, but at least four vaccines are in development. All four were effective in animal studies. Small, early-stage clinical trials in humans have shown promising results. However, study participants who received the Marburg vaccine were not exposed to the virus to test how well the vaccine protected. Two other vaccines against Ebola virus may also be effective against Marburg virus.
Rwandan researchers are preparing to submit a phase 3 trial protocol to Rwanda’s Ethics Committee for approval. This will be an opportunity to accelerate the development of vaccines and treatments for Marburg virus and conduct large-scale trials in real-world outbreaks.
If trials move quickly, the current crisis could lead to scientists identifying a vaccine that will protect millions of people in the coming years.
A ray of hope for the slim vaccine
The WHO, Rwandan public health authorities, and a group of scientists and institutions working on vaccines met earlier this week to discuss quickly starting vaccine trials to help quell the ongoing outbreak.
Among the vaccine candidates in development, the one that seems most likely to be tested in Rwanda is the cAd3-Marburg vaccine developed by the National Institute of Allergy and Infectious Diseases (NIAID). Last year, NIAID first tested the vaccine’s safety in humans in a Phase 1 clinical trial.
Forty healthy adults were given one of two different doses of the vaccine, and side effects were observed and whether their immune systems produced antibodies. This indicates that the vaccine is effective if the individual is exposed to the virus. The vaccine was deemed safe, with 95% of participants developing antibodies four weeks after vaccination. However, because the participants had not been exposed to the virus, the vaccine’s actual effectiveness could not be assessed.
Earlier this year, the Sabin Vaccine Institute began Phase 2 clinical trials of the NIAID vaccine in Uganda and Kenya. Healthy adults will be randomly assigned to receive either the vaccine or a placebo and then monitored again for adverse reactions and antibody responses. Additionally, participants will not be infected with Marburg virus during this trial.
Typically, if Phase 2 trials are successful, scientists move on to large-scale Phase 3 trials, where they roll out the vaccine to large groups of people and observe the effects when individuals are exposed to the virus. The emergency situation in Rwanda could accelerate this process. Even if Phase 2 results are not yet available, scientists may proceed with Phase 3 trials.
Researchers and health officials have been preparing for this. Earlier this year, the WHO and scientists from 17 countries at risk of Marburg virus outbreaks developed a protocol to test both vaccines and treatments during the outbreak. The phase 3 clinical trial in Rwanda will follow a ring vaccination strategy in which contacts of infected people are vaccinated. There are two groups in the trial: a group of contacts who will be vaccinated immediately, and a “delayed” group who will probably be vaccinated later. Researchers will then compare the effects of the vaccine between the two groups.
Scientists may also test treatments that use antibodies, proteins made by the immune system, against viruses to kill viruses and other pathogens. One study found that treating animals such as guinea pigs and monkeys with these antibodies after being infected with Marburg virus prevented severe illness and death. During previous Ebola virus outbreaks, health workers successfully treated patients with antibody cocktails.
The WHO’s ethics committee has already approved trial protocols for vaccines and antibody treatments, a spokesperson told Vox. The next step is for these protocols to be approved by Rwanda’s national ethics committee. Two Rwandan researchers have been selected to lead these efforts in the country.
One more hurdle remains. The total number of vaccine doses currently available is less than 2,000. As of April 2023, the Sabin Vaccine Institute had produced about 850 of those doses. Oxford University is developing another Marburg vaccine and has around 1,000 doses available. Larger scale production may be needed, especially if the infection spreads further. The Sabin Vaccine Institute and the University of Oxford both have existing manufacturing capacity for the Marburg vaccine and have previously informed the WHO that additional doses can be produced quickly.
The Marburg disease outbreak comes amid a surge in mpox outbreaks in the region, leading the World Health Organization to declare it an international health emergency in August. The Democratic Republic of the Congo, which borders Rwanda, is the epicenter of the mpox epidemic. (The Democratic Republic of the Congo has not yet recorded the Marburg incident.)
Fortunately, Rwanda has one of the strongest public health systems in Africa and has only reported a few cases of mpox. An estimated 90 percent of the population has health insurance, much higher than most of its neighboring countries. Rwanda also has a strong infectious disease surveillance system and a history of successfully containing outbreaks.
This makes it an ideal environment to test the Marburg vaccine during the current outbreak, if public health authorities can act quickly. The race is on.
I read 1 article last month
At Vox, we believe everyone can help make sense of and shape our complex world. Our mission is to create clear, accessible journalism that inspires understanding and action.
If you share our vision, please consider supporting our work by becoming a Vox Member. Your support allows Vox to provide a stable, independent source of funding for our journalism. If you’re not ready to become a member, making even a small donation means a lot to support our sustainable model of journalism.
Thank you for joining our community.
Swati Sharma
vox editor in chief