research participants
The study began in May 2021 and involved 335 people (194 staff and 141 residents) without a history of SARS-CoV-2 infection from six nursing homes in Toyama Prefecture, Japan19. By May 2023, two months after the fifth vaccination, 111 participants (78 residents and 33 staff) remained in the study. Reasons for excluding 224 participants included study discontinuation, staff retirement, failure to receive the fifth vaccination, failure to complete questionnaires, and death of the participant.
Of the remaining 111 participants, 95 participants had no experience with SARS-CoV-2, consisting of 26 staff (median age 51 years) and 69 residents (median age 88 years). , the incidence of local and systemic AEs was evaluated. SARS-CoV-2-uninfected cases were defined as having no confirmed coronavirus disease (COVID-19) and a negative anti-nucleocapsid protein IgG result. The definition of a BTI case has been outlined previously19. Briefly, BTI cases were detected by reverse transcription-polymerase chain reaction (RT-PCR), immunochromatographic testing for SARS-CoV-2, or anti-inflammatory testing before vaccination, 2 months and 5 months after the second dose. It was defined based on a positive result for nucleocapsid protein IgG. , 3rd and 5th vaccination19.
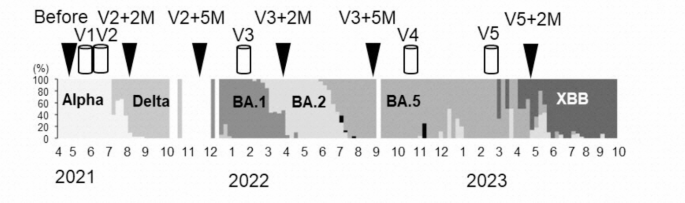
All participants received the first dose of the novel coronavirus disease (COVID-19) mRNA vaccine BNT162b2 (Pfizer/BioNTech, 30 μg per dose) targeting the Wuhan strain between April and June 2021. Received 2 doses of vaccination. After that, 32 participants were eligible for the third vaccination. From January to March 2022, 11 participants received the BNT162b2 vaccine and 79 received the mRNA-1273 vaccine (Moderna, 50 μg per dose). For the fourth vaccination, 11 people received the BNT162b2 vaccine and 100 people received the mRNA-1273 vaccine during July 2022. For the fifth vaccination, 30 participants received either the Wuhan/BA.1-adapted BNT162b2 mRNA vaccine23 and 81 received either the Wuhan/BA.4/BA.5-adapted BNT162b2 mRNA vaccine24 or the bivalent mRNA vaccine. was inoculated. Bivalent vaccines contained ancestral SARS-CoV-2 strain components and components of either the BA.1 or BA.4/BA.5 subvariants. The safety profile of these bivalent vaccines was comparable to that of monovalent vaccines (BNT162b2 or mRNA-1273) 23 , 24 , 25 , 26 , 27 . In our study, we did not randomly select nursing home participants who participated in the survey. The choice of vaccine type was determined by the policy of the municipality and facility for residents, by the policy of the municipality and facility for staff for the first to third vaccinations, and by the individual for the fourth and fifth vaccinations. .
Blood samples were collected in May-June 2021 before the first COVID-19 vaccination, and then 2 and 5 months after the primary series vaccination (August-September 2021 and August-September 2021, respectively). Collected from November to December 2021). Subsequent blood draws will be taken 2 and 5 months after the third booster vaccination (March-June 2022 and June-September 2022, respectively) and after the fifth vaccination. It was conducted 2 months later (March-April 2023) (Figure 1). . Participants received their fifth dose before their scheduled blood draw, so a post-fourth dose blood sample could not be collected.
This study received approval from the Toyama Institute of Health Ethics Review Board (approval number #R4-14) and complies with the principles outlined in the Declaration of Helsinki. Informed consent was obtained from all participants or their representatives.
Genotyping of SARS-CoV-2
As part of the country’s new coronavirus infection surveillance activities, clinical samples such as nasopharyngeal swabs and saliva that had positive reactions in RT-PCR and antigen tests at hospitals in Toyama Prefecture were collected at the Toyama Health Research Institute. . A total of 2,223 samples were subjected to genomic analysis from March 2021 to October 2023. Whole genome sequencing and variant classification were performed according to previously established protocols 19 .
Adverse events after vaccination
A questionnaire was distributed to study participants to assess the presence of local or systemic AEs over a 7-day period following each dose of COVID-19 mRNA vaccination. Local AEs included pain, redness, and swelling at the injection site, and systemic AEs included fever, fatigue, headache, chills, vomiting, diarrhea, myalgia, arthralgia, and anaphylactic shock. The severity of non-life-threatening AEs was graded into no AE, mild AE, moderate AE, and severe AE, respectively, according to a previously established scale. Injection site pain, fatigue, headache, chills, myalgia, and arthralgia were evaluated according to the following scale: Mild, does not interfere with activity. Moderate, inhibits activity. Severe cases interfere with daily activities. Redness and swelling were measured according to the following scale: Mild, 2.0-5.0 cm in diameter. Moderate, diameter > 5.0 to 10.0 cm. Severe cases with diameter greater than 10.0 cm. Categories of fever are designated as follows: Additional scales are: vomiting (mild, 1 to 2 times in 24 hours; moderate, more than 2 times in 24 hours; severe, requiring intravenous hydration); and diarrhea (mild, 2 to 3 4 to 5 moderately loose stools in 24 hours, or 6 or more loose stools in 24 hours if severe.35. The AE percentage indicated the frequency of each of the above AEs. Nursing staff were responsible for collecting AE information from study participants, including residents with communication difficulties. Nursing home staff conducted daily AE assessments for each sign or symptom in residents for 7 days after each vaccination.
Ninety-five participants (26 staff and 69 residents) who had never had SARS-CoV-2 underwent analysis of AE rates. No study participants required hospitalization after vaccination.
Neutralizing activity against SARS-CoV-2 pseudotype virus
Pseudotyped vesicular stomatitis virus (VSV) harboring the SARS-CoV-2 Spike (S) protein was generated according to established procedures 36 . Expression plasmids for truncated S proteins of SARS-CoV-2 variants, namely pCAGG-pm3-SARS2-SHu-d19_B.1.1.529.5 (Omicron BA.5 subvariant) and pCAGG-pm3-SARS2-SHu-d19_XBB1.5 (Omicron) XBB.1.5 subvariant) was generously provided by Drs. C. Ono and Y. Matsuura of the Research Institute for Microbial Diseases, Osaka University. SARS-CoV-2 pseudotyped VSV (SARS-CoV-2pv) was stored at −80 °C until use. Neutralizing activity was determined during chemiluminescent reduction of SARS-CoV2pv with S protein, Omicron BA.5 (B.1.1.529.5), and Omicron XBB.1.5 from an ancestral strain (Wuhan) according to previously established protocols. was evaluated using the sum test19,36.
SARS-CoV-2 IgG ELISA
Anti-RBD IgG and anti-nucleocapsid IgG ELISAs were performed as previously described.
statistical analysis
Postvaccination AEs stratified by age, sex, mean anti-RBD IgG value, and geometric mean NT were analyzed using IBM SPSS Statistics 27.0 (IBM, Armonk, NY). NT values were logarithmically transformed. Statistical significance was defined at p < 0.05. Missing data were excluded and only datasets where all variables were available were used. The Mann-Whitney U test or Friedman test was used to compare nonparametric variables (antibody titers and age) between groups (staff and residents, vaccination history). Pearson's chi-square test was utilized to compare the proportion of AEs by vaccine dose. Statistical power for the initial target sample size was not calculated in this study.