Study participants
In an observational study (CS041), a total of 41 PLWH were recruited before SARS-CoV-2 mRNA vaccination and stratified based on peripheral CD4 T-cell count as Immune Non-Responder ( < 350 per μL, n = 21, INR) and Immune Responder ( > 350 per μL, n = 20, IR), as shown in Supplementary Tables S1 and S2 and Supplementary Fig. 7. Longitudinal follow-up of vaccinated PLWH allowed the identification of patients suffering from COVID-19 (HIV BTI, n = 16), by positive serology for anti-nucleocapsid IgG. Time-points for BTI for individual PLWH were defined according to the patient’s self-declaration of COVID-19, verified by serological measures. 6/21 INR had transient CD4 > 400 cells/µL during the last years, but only 3 INR had CD4 count >500 cells/µL. We defined a cut-off of 2000 BAU/mL, 1 month after previous mRNA vaccine doses, to identify vaccine-induced seroconversion (standardized WHO units). PLWH received essentially mRNA-based vaccines (BNT162b2, Pfizer-BioNTech or mRNA-1273, Moderna) except one patient for the first dose of vaccine (ChAdOx1, AstraZeneca).
Peripheral blood mononuclear cells (PBMC) samples were collected from PLWH after dose 2 (n = 41), and after BTI (n = 16).
The anti-SARS-CoV-2 humoral and cellular immune responses in non-hospitalized PLWH were compared to three control groups: (1) the immune responses observed in HIV-negative hospitalized COVID-19 patients with moderate to severe disease recruited in 2020 (Cohort 1, n = 20 with follow-up from day 7 up to one year) with the Wuhan wild type SARS-CoV-2 variant. See Supplementary Table S3 for cohort details. (2) A cohort with severe COVID-19 recruited in the period 2020-21 infected with SARS-CoV-2 Wuhan and Alpha variants (Cohort 2, n = 17) (Supplementary Table S3). (3) Vaccinated non-hospitalized patients with BTI with either Delta or Omicron (n = 37), (Supplementary Table S4). All patients aged ≥18 years admitted to the hospital with polymerase chain reaction (PCR)-confirmed SARS-2-CoV-2 infection were eligible for inclusion. Blood samples were obtained from each patient within 48 h of admission and up to 10 days during hospitalization, as well as at 3-month and 12-month follow-ups in a subset of patients for cohorts 1 and 2. From December 2021, adults with positive SARS-CoV-2 RT-PCR test, with or without positive Omicron or Delta variant PCR on oro-nasopharyngeal specimens (i.e., verified or suspected Omicron/Delta variant cases, respectively), and symptomatic household members of suspected Omicron cases were consecutively recruited to a prospective cohort study (a joint venture between The Norwegian Corona Cohort (NCT04320732) and the Norwegian SARS-CoV-2 study (NCT04381819)). Pre-2019 pandemic or pre-vaccination healthy donors (HD), as well as SARS-CoV-2 naïve healthy donors after 2nd doses of vaccines (n = 19), were used as controls. Patients with sepsis but without HIV or SARS-CoV-2 infection admitted to the intensive care unit (ICU) were used as a validation cohort for the inflammatory response (n = 16, Supplementary Table S5). Sepsis patients (n = 16) conforming to Sepsis-3 criteria, were sampled at hospital admission90.
Ethical considerations
All participants provided written informed consent. The study was approved by the Regional Committees for Medical and Health Research Ethics in South-Eastern Norway (REK #106624 for the Norwegian SARS CoV2 cohort and REK 1.2007.83 and 2015/629 for the PLWH cohort).
Sample preparation
Each sample consisted of two frozen aliquots with an average cell number of 10 million cells and viability above 95% per donor. Samples were thawed at 37 °C and immediately transferred into a complete RPMI medium (10% FCS, 1% penicillin /streptomycin, glutamine, 10 mM HEPES). After the first wash, thawed cells were incubated for 15 min at room temperature with DNAse (STEMCELL). Live cells were purified by removing dead cells using a column-based magnetic depletion approach according to the manufacturer’s recommendations (Miltenyi). Vaccinated healthy donor PBMCs matched for at least one of the donor HLA alleles were included in each experiment as a control for specific T-cell identification. VeriCells were included in each experiment as a control for phenotypic markers.
HLA typing
PLWH and HD were pre-typed by flow cytometry for A02, A24, and B07 expression. Frozen PBMCs from individuals positive for specific HLA were subsequently stained with the corresponding Dextramers/ Tetramers Class I restricted (HLA-A*01:01, HLA-A*02:01, HLA-A*24:02, and HLA-B*07:02) as described33. The non-SARS-CoV-2-related viruses were used as an internal control for the same individuals (CMV, EBV, Influenza, see Supplementary section).
Inflammatory markers
The following enzyme-linked immunosorbent assay (ELISA) kits were used according to manufacturer protocols. From R&D Systems: Human CD14 DuoSet ELISA (DY383), Human CD163 DuoSet ELISA (DY1607), Human LBP DuoSet ELISA (DY870-05), Human Galectin-9 DuoSet ELISA (DY2045), Human GDF-15 Quantikine ELISA Kit (DGD150), Human CXCL4/PF4 Quantikine ELISA (Kit DPF40), Human IFN-alpha (41100); from Ebioscience: Human MPO Instant ELISA Kit (BMS2038INST); from Thermo Scientific: Invitrogen novex IP 10 Human ELISA Kit (KAC2361); from Abcam Human C-Reactive Protein/CRP (Ab99995); from MyBioSource: Human zonulin ELISA Kit (MBS706368); from Meso Scale diagnostics: human Calprotectin (F21YB-3).
Olink® Target 96 inflammatory panel
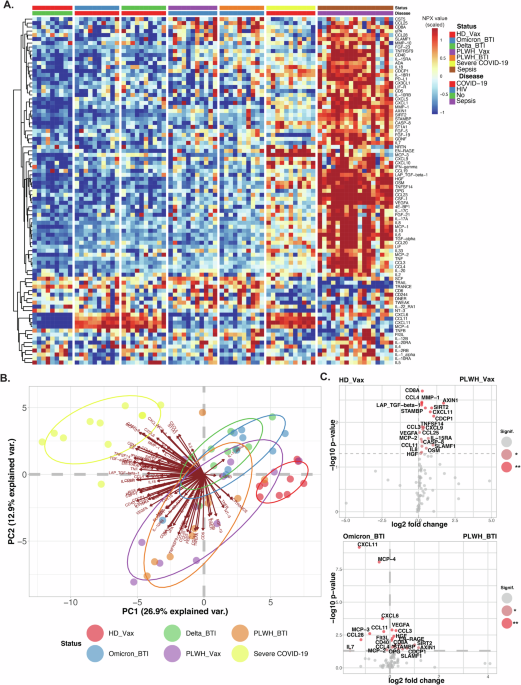
The systemic inflammation of PLWH, after vaccine dose 2 or BTI and controls (vaccinated HD, BTI, or severe COVID-19 patients) was determined by analyzing CPT plasma samples by Olink Proteomic Proximity Extension Assay (PEA) technology. The Olink® Target 96 inflammatory panel was used on an Olink® Signature Q100 instrument according to the manufacturer´s instructions.
Flow cytometry
Cryo-preserved PBMCs were enriched for live cells by magnetic depletion of dead cells (Dead cells removal microbeads, Miltenyi) in the presence of Citrate buffer and stained with antibody panels to quantify and characterize the phenotype of specific T-cell responses directed against Spike peptides (two million cells per sample) and B-cell responses to RBD or Spike protein (one million cells per sample). Cells were acquired on a BD FACSymphony (BD Biosciences) or Attune NxT (ThermoFisher).
MAbs were for BD FACSymphony: BB515 Mouse Anti-Human CD279 (PD-1) Clone EH12.1, BD Biosciences, PerCP-eFluor 710, KLRG1 Monoclonal Antibody (13F12F2), eBioscience, PE/Cyanine7 anti-human GPR56, Clone CG4, Nordic Biosite, Alexa Fluor 700 anti-human CD244 (2B4), clone C1.7, Nordic Biosite, APC/Cyanine7 anti-human HLA-DR, clone L243, Nordic Biosite, BV480 Rat Anti-Human CXCR5 (CD185) (Clone: RF8B2) BD Biosciences, BB515 Mouse Anti-Human CD38, clone, HIT2 BD Biosciences, Brilliant Violet 570™ anti-human CD3, Nordic Biosite, Brilliant Violet 605, CD127 Mouse anti Human, Clone HIL 7R M21, BD Biosciences, Brilliant Violet 650, CD161 Mouse anti Human, clone: DX12, BD Biosciences, BV711 Mouse Anti-Human TIM-3 (CD366), clone 7D3, BD Biosciences, BV750 Mouse Anti-Human CD8, clone SK1, BD Biosciences, Brilliant Violet 785™ anti-human CD57 Recombinant, clone QA17A04, Nordic Biosite, BV421 Mouse Anti-Human CD319 (CRACC), BD Biosciences, BUV395 Mouse Anti-Human TIGIT, clone 741182, BD Biosciences, Live/dead™ Fixable Blue Dead Cell Stain Kit, for UV excitation, ThermoFisher Scientific, BUV563 Mouse Anti-Human CD45RO, clone UCHL1, BD Biosciences, BUV615 Mouse Anti-Human CD95, clone DX, BD Biosciences, BUV661 Mouse Anti-Human CD4, clone SK3, BD Biosciences, BUV737 Mouse Anti-Human CD38, clone HB7, BD Biosciences, BUV805 Mouse Anti-Human CD27, clone L128, BD Biosciences. VeriCells PBMC (BioLegend) were included as controls.
Frequency values were calculated based on the percentage of the parent immune cell population, and phenotypic markers were gated individually for each sample and calculated as % of positive cells. High-dimensional phenotypic profiles and sample distributions were shown using uniform manifold approximation and projection. Data analysis was performed using CYTOGRAPHER® (ImmunoScape cloud-based analytical software), custom R-scripts, GraphPad Prism (GraphPad Software), and FlowJo v10 software (BD Life Sciences). Statistical significance was set at a threshold of *p < 0.05, **p < 0.01, and ***p < 0.001.
Detection of specific memory CD8 T cells
Antigen-specific CD8 T cells were detected by peptide: HLA multimers as described33 (see Supplementary section and Supplementary Table S7 for an overview). The peptides listed below are referenced individually in the Supplementary Section. Spike-Specific CD8 were detected using PE-conjugated Dextramers (Immudex) targeting Spike and restricted to HLA-A*0101 (LTDEMIAQY), HLA-A*0201 (YLQPRTFLL), HLA-A*2402 (QYIKWPWYI), and HLA-B*0702 (SPRRARSVA). The panel was expanded using Flex-T tetramer according to the manufacturer´s instructions (BioLegend). We UV-exchanged peptides for Spike epitopes restricted to HLA-A*0101 (YTNSFTRGVY), HLA-A*0201 (LITGRLQSL and RLNEVAKNL), HLA-A*2402 (NYNYLYRLF), and HLA-B*0702 (APHGVVFL) and tetramerized with Streptavidin-PE (Biolegend). A similar approach was performed for non-Spike derived epitopes, including HLA-A*0101 (ORF3a, FTSDYYQLY, and ORF1ab, TTDPSFLGRY), HLA-A*0201 (ORF3a, LLYDANYFL), HLA-A*2402 (ORF3a, VYFLQSINF), and HLA-B*0702 (Nucleoprotein, SPRWYFYYL) and tetramerized with Streptavidin-APC (Biolegend). CMV-and EBV/FLU-specific CD8 T cells were generated similarly and tetramerized using Streptavidin-PECF594 (Biolegend) and Streptavidin-PE-Cy5, respectively. CMV-derived epitopes were for HLA-A*0101 (DNA polymerase processivity factor, VTEHDTLLY), HLA-A*0201 (65 kDa phosphoprotein, NLVPMVATV), HLA-A*2402 (65 kDa phosphoprotein, QYDPVAALF), and HLA-B*0702 (65 kDa phosphoprotein, RPHERNGFTVL) and EBV derived epitopes were for HLA-A*0201 (EBV LMP2, FLYALALLL) and HLA-B*0702 (EBV antigen 3, RPPIFIRRL). A Flu peptide was for HLA-A*0101 (Nucleoprotein, Influenza A virus CTELKLSDY). All peptides were ordered from Genscript with a purity above 85% by HPLC purification and mass spectrometry. Lyophilized peptides were reconstituted at a stock concentration of 10 mM in DMSO.
Antigen-specific multimer CD8 T cells were identified by fine manual gating. The designation of bona fide antigen-specific T cells was further dependent on (a) the detection cut-off threshold ( ≥ 5 events to be detected), (b) the background noise (frequencies of specific CD8 T cells must be greater than frequencies from the corresponding CD4 T-cell population) as unbiased objective criteria for antigen-specificity assessment. Spike and non-Spike Dextramers staining have been extensively validated in COVID-19 convalescent patients and in SARS-CoV-2 vaccinated healthy donors during longitudinal follow-up. The peptides are referenced individually in the Supplementary Section.
Detection of SARS-CoV-2-specific memory B cells
Spike-specific B cells from thawed PBMCs were detected using sequential staining of biotinylated Recombinant WT SARS-CoV-2 Spike-Trimer and RBD (HEK) (Miltenyi) conjugated with streptavidin-PE or streptavidin-BV786 respectively and combined with probes already conjugated with Alexa Fluor 647 for Spike RBD B1.1, 529 (AFR11056, R&D Systems) and conjugated with Alexa Fluor 488 for Full-length Spike B1.1, 529 protein (AFG11061, R&D Systems,). Commercial probes were already conjugated with Alexa Fluor 647 for Spike RBD B1.1, 529 (AFR11056, Gly339Asp, Ser371Leu, Ser373Pro, Ser375Phe, Lys417Asn, Asn440Lys, Gly446Ser, Ser477Asn, Thr478Lys, Glu484Ala, Gln493Arg, Gly496Ser, Gln498Arg, Asn501Tyr, Tyr505His, R&D Systems) and conjugated with Alexa Fluor 488 for Full-length Spike B1.1, 529 protein (AFG11061, Ala67Val, His69del, Val70del, Thr95Ile, Gly142Asp, Val143del, Tyr144del, Tyr145del, Asn211del, Leu212Ile, ins214Glu-Pro-Glu, Gly339Asp, Ser371Leu, Ser373Pro, Ser375Phe, Lys417Asn, Asn440Lys, Gly446Ser, Ser477Asn, Thr478Lys, Glu484Ala, Gln493Arg, Gly496Ser, Gln498Arg, Asn501Tyr, Tyr505His, Thr547Lys, Asp614Gly, His655Tyr, Asn679Lys, Pro681His, Asn764Lys, Asp796Tyr, Asn856Lys, Gln954His, Asn969Lys, Leu981Phe) (Arg682Ser, Arg685Ser, Lys986Pro, Val987Pro, R&D Systems,). 2 × 106 cryo-preserved PBMC samples were transferred in a 96-well U-bottom plate. Cells were first stained with Fc block (BD Biosciences) for 15 min at room temperature. Cells were then washed and stained with probe master mix containing 100 ng Spike-A488, and 25 ng RBD-A647 for 1 h at 4 °C. Following incubation with antigen probes, cells were washed twice and stained with Blue Live Dead (Thermo Fischer) for 10 min at room temperature. Cells were washed again and stained with antibodies according to manufacturer protocols: BUV805-Mouse Anti-Human CD7, clone M-T701 BD Biosciences, BUV805-Mouse Anti-Human CD14, clone M5E2, BD Biosciences, BV711-Mouse Anti-Human CD19, clone HIB19, BD Biosciences, BUV395-Mouse Anti-Human CD20, clone 2H7, BD Biosciences, BUV737-Mouse Anti-Human CD21, clone B-ly4, BD Biosciences, BUV615-Mouse Anti-Human CD24, clone ML5, BD Biosciences, A700-Mouse Anti-Human CD27, clone L128, BD Biosciences, PE-CF594-Mouse Anti-Human CD38, clone HIT2, BD Biosciences, PecyPE-Cy-7-Mouse Anti-Human CD71, clone CY1G4, BD Biosciences, BV605-Mouse Anti-Human IgD, clone IA6-2, BD Biosciences, Percp.5PerCP-Cy5.5 Mouse Anti-Human IgM, clone MHM-88, Biolegend, BV421-Mouse Anti-Human IgG, clone G18-145, BD Biosciences, APC-H7-Mouse Anti-Human HLA-DR, clone L243, Biolegend, and BV480-Rat Anti-Human CXCR5, clone RF8B2, BD Biosciences for 30 min on ice. Cells stained with the Spike Trimer were fixed with the transcription factor buffer (Thermo Fischer) and intra-cellularly stained for IRF4 (eFluor660, clone 3E4, Thermo Fischer) and Blimp-1 (PE-CF594, clone 6D3, BD Biosciences). Cells stained with RBD and full Spike probes were fixed overnight in 1% PFA. Samples were acquired on BD FACSymphony.
In vitro stimulation assays
Thawed cells were stimulated for 16 h with SARS-CoV-2 PepTivator Spike and non-Spike peptides consisting of 15-mer sequences with 11 amino acid overlaps (Wuhan-Hu-1, i.e., wild-type WT. Miltenyi Biotec) as described33. In brief, thawed cells were stimulated for 16 h with SARS-CoV-2 PepTivator Spike protein peptides consisting of 15-mer sequences with 11 amino acid overlaps (Wuhan-Hu-1, i.e. wild type WT. Miltenyi Biotec) as described (1). For non-Spike (WT) responses, cells were stimulated with Nucleoprotein (PepTivator SARS-CoV-2 Prot N) and Membrane protein (PepTivator SARS-CoV-2 Prot M), consisting of 15-mer sequences with 11 amino acid overlaps in addition to the 4 ORF1ab/Orf3a peptides in Supplementary Table S7, i.e., stimulated with M + N + O. Alternatively, cells were stimulated with 88 pooled WT immunodominant oligopeptides from the whole proteome (PepTivator SARS-CoV-2 Select, Miltenyi Biotec) consisting of peptides from structural proteins (S, M, N) as well as non-structural proteins (O).
Thawed cells were also stimulated for 16 h with PepMixTM HIV-1 (Con B gag motif, JPT) consisting of a Pool of 123 peptides derived from a peptide scan (15mers with 11 aa overlap) through Con B gag motif of Human Immunodeficiency Virus (HIV).
Peptide stimulation was performed on 1 million PBMCs per condition in the presence of costimulatory antibodies against CD28 and CD49d (BD Biosciences) and Brefeldin-A (10 μg/mL, Millipore Sigma). SARS-CoV-2-specific T cells were identified by dual expression of CD40L (CD154) and CD137, interferon-gamma (IFN-γ), interleukin-2 (IL-2), or tumor necrosis factor (TNF) for CD4 T cells and by dual expression of IFN-γ and TNF or CD137 and IL-2, TNF or IFN-γ for CD8 T cells.
Serology
A multiplexed bead-based flow cytometry assay, referred to as microsphere affinity proteomics (MAP), was adapted for the detection of WT and VOC SARS-CoV-2 Spike and the receptor-binding domain (RBD) antibodies or anti-viral IgG antibodies (anti-EBV, anti-FLU, anti-Rhinovirus) as described91,92,93,94.
Statistics
Comparative analyses of frequencies of cell subsets and marker expression are presented by GraphPad Prism version 10.1. The difference between the control and test group was tested using the Mann–Whitney U-test for unpaired data and the Wilcoxon test on paired samples for the comparison between unstimulated and peptide-stimulated samples. Tests were two-sided. Values of p < 0.05 were considered statistically significant. Correlations were calculated with Pearson’s test. A correlation matrix was calculated comparing phenotypic and serological marker variables in a pairwise fashion, using the corr. test function from the psych CRAN package; the corrplot package was subsequently used to graphically display the correlation matrix. The resulting p-values were adjusted for multiple testing using the Bonferroni method. Pearson’s correlation coefficients were indicated by a heat scale, whereby the red color shows a positive linear correlation, and the blue color shows a negative linear correlation. The volcano plots and the correlation matrix were integrated as a package in CYTOGRAPHER®, ImmunoScape cloud-based analytical software. The SPICE (Simplified presentation of incredible complex evaluations) software was used to determine the polyfunctionality of antigen-specific T cells. A permutation test from SPICE software was used to compare the distribution of cell populations for each pair of pie charts. Values of p < 0.05 were considered statistically significant.