Formulation of the monovalent, trivalent, and quadrivalent modRNA-HA vaccines
ModRNA-HA vaccines encoding full-length HA proteins of WHO-recommended strains for cell culture- or recombinant-based vaccines for use in either the 2021–2022 (A/Wisconsin/588/2019 (H1N1), A/Cambodia/e0826360/2020 (H3N2), B/Washington/02/2019 (B/Victoria), and B/Phuket/3073/2013 (B/Yamagata))26 or 2022–2023 (A/Wisconsin/588/2019 (H1N1), A/Darwin/6/2021 (H3N2), B/Austria/1359417/2021 (B/Victoria), and B/Phuket/3073/2013 (B/Yamagata))27 northern hemisphere influenza seasons were individually formulated and prepared as either monovalent, trivalent, or quadrivalent formulations as previously described for the BNT162b2 modRNA vaccine17. Stock concentrations of modRNA-HA were diluted in saline to achieve the desired dose for vaccine administration in animals.
In vitro expression of HA from the influenza modRNA-HA vaccine
Individually LNP-formulated modRNAs encoding full-length HA from H1N1, H3N2, B/Victoria, or B/Yamagata strains were diluted in Opti-MEM (Thermo Fisher, Cat #31985062) and directly added to a HEK-293T (CRL-3216, ATCC) cell monolayer at four RNA dose levels (62.5, 31.1, 15.6, and 7.8 ng/well). The input amount of mRNA encoding the strain-specific HA was the same between modRNA formulations (e.g., different amounts of mIRV and qIRV were applied to cells to achieve a final concentration of 62.5 ng/well of mRNA encoding the B/Phuket/3073/2013 HA). Opti-MEM media alone was used as a negative control. Protein expression was measured with a flow cytometer (BD FACS Fortessa) using in-house generated rabbit polyclonal antibodies raised against each of the following strains: A/Wisconsin/588/2019 (H1N1), A/Darwin/6/2021 (H3N2), B/Austria/1359417/2021 (B/Victoria), or B/Phuket/3073/2013 (B/Yamagata), followed by a secondary anti-rabbit antibody conjugated to Alexa-Fluor 488 (Invitrogen, Cat # A-11008). The percentage of live cells expressing the strain-specific HA protein was enumerated and expression was measured by quantifying the number of live cells that had a positive signal for bound anti-HA antibody.
Animals
Mouse immunogenicity studies utilized female BALB/c mice (The Jackson Laboratory) that were first immunized between 7-13 weeks of age. NHP immunization studies utilized female rhesus macaques (Macaca mulatta) and cynomolgus macaques (Macaca fasicularis) from the Pfizer colony at Pearl River, NY that were co-housed in standard quad caging. Rhesus and cynomolgus macaques were immunized at 5.5 and 13.5–15.5 years of age, respectively. Animal studies were performed at Pfizer, Pearl River, NY, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC).
For mIRV and qIRV toxicology studies, male and female Wistar Han rats, 11 weeks of age at the dosing study start, were supplied by Charles River Laboratories. Toxicology studies were conducted at Pfizer, Groton, CT according to GLP and OECD guidelines. Animals were offered Certified Irradiated Rodent Diet 2916 C (Envigo Teklad Global Diet) and locally sourced water ad libitum, except when fasting was conducted prior to clinical pathology collections or euthanasia. Animals were socially housed throughout the study in ventilated Tecniplast double decker polyphenylsulfone cages with Enrich-n’Pure® (The AndersonsP, Inc.). Environmental conditions across studies were set to maintain relative humidity ranging from 30% to 70% and temperature ranging from 68 °F to 79 °F with room lighting set to provide a 12-h light/dark cycle.
All procedures performed on animals were in accordance with regulations and established guidelines and were reviewed and approved by an Institutional Animal Care and Use Committee.
Mouse immunization study design
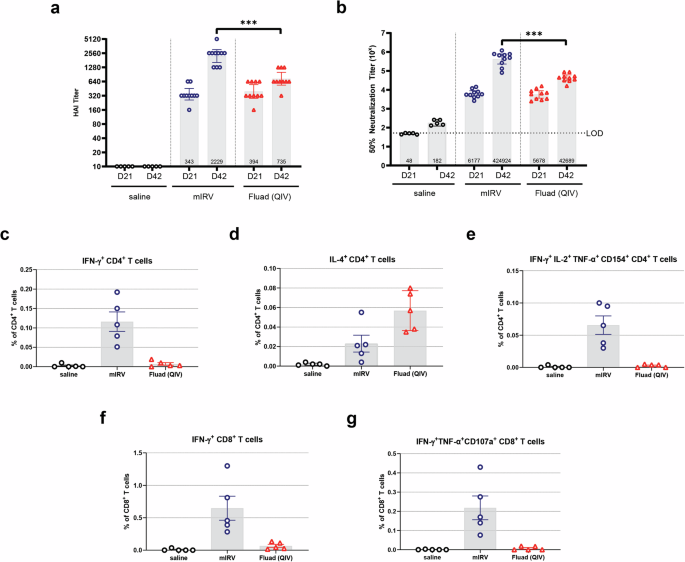
Experimental vaccine groups each contained 10 female BALB/c mice. Control groups consisted of 5 or 10 female BALB/c mice that were administered 50 µL of saline intramuscularly (IM). For all studies, mice were immunized IM twice, on Days 0 and 28. Groups receiving modRNA-HA vaccines received 0.2 µg of each mRNA HA construct. Thus, mIRV vaccines were administered at a 0.2 µg dose in a 50 µL volume; tIRV was administered at a 0.6 µg dose (0.2 µg/HA) in a 50 µL volume; and qIRV vaccines were administered at a 0.8 µg dose (0.2 µg /HA) in a 50 µL volume. An undiluted comparator QIV (Fluad®) was administered IM at a final concentration of 2.4 µg in a 20 µL dose volume (1/25th of the human dose), also on Days 0 and 28. Whole blood was collected on Day 21 (3 weeks post-dose 1) and Day 42 (2 weeks post-dose 2) and evaluated for levels of functional anti-HA antibodies by serology. Splenocytes were isolated on Day 42 (study end) to measure the T cell-mediated immune response following immunization.
NHP immunization study design
Rhesus and cynomolgus macaques (3 animals/group) were each immunized IM with a 30 µg dose of mIRV (A/Wisconsin/588/2019) in a 0.5 mL total volume. Whole blood was collected on Days -7 (pre-vaccination), 7, 21, 28, 35, 42, 77, 105, 133, and 168 and evaluated for levels of functional anti-HA antibodies by serology. PBMCs were isolated on Days -7 (pre-vaccination), 7, 35, 42, 77, 105, 133 and 168 to measure T cell responses following immunization.
Toxicology study design
For both toxicology studies, male and female Wistar Han rats (15/sex/group) were randomly assigned to Groups 1 or 2, and doses were administered IM by 2 separate injections on Days 1 and 15. Group 1 was administered sterile saline. Group 2 was administered with either 34 µg mIRV or 30 µg qIRV (7.5 µg/HA). A subset of animals (10/sex/group) was euthanized 2 days after the second dose (Day 17), while the remaining animals (5/sex/group) underwent an approximate 3-week recovery and then were euthanized (Day 38–39). Serum samples were collected from each animal prior to dose initiation and on Day 17 (dosing phase) and Day 21 (recovery phase) for analysis of hemagglutination inhibition, to confirm in parallel functional immunogenicity of the mIRV and qIRV under toxicological observation.
Samples for clinical pathology analysis in toxicology studies (hematology, coagulation, clinical chemistry, acute phase proteins and urinalysis) were collected on Days 3 (nonterminal; hematology, clinical chemistry, and acute phase proteins A1AGP and A2M only), 17 (terminal), and 39 (terminal), from overnight fasted animals. For non-terminal collections, hematology was assessed in the first 7 animals/sex/group and clinical chemistry was assessed in the last 8 animals/sex/group. Phlebotomy sites included the jugular vein (non-terminal bleed) or aorta under isoflurane anesthesia followed by exsanguination (terminal bleed). Blood samples were collected into appropriate tubes (K2EDTA for hematology, 3.2% sodium citrate for coagulation, serum separator for clinical chemistry and acute phase proteins).
Animal blood collection and splenocyte isolation
For mouse immunization studies, the interim bleed was conducted using a submandibular bleeding technique. At the study end, blood was collected via cardiac puncture (terminal bleed). Whole blood tubes remained at room temperature (RT) for at least 30 min prior to centrifuging at 10,000 RPM for 3 min for sera collection. Samples for HAI and MNT assays were treated using a receptor destroying enzyme (RDE) kit (Accurate Chemical), heat inactivated and pre-adsorbed with turkey red blood cells (RBCs). Samples were stored at −80 °C until testing. At the study end, spleens were collected from 5 mice per group and separately placed in a 70 µm cell strainer (Fisher) immersed in 7 mL of complete RPMI (cRPMI: 10% FBS/RPMI; Pen-Strep; Sodium pyruvate; HEPES; MEM-NEAA; Amphotericin B) per well of a 6-well plate. Plates were maintained on ice during transit and before processing for single cell suspension. Spleens were homogenized, subjected to RBC lysis, and passed through a cell strainer to remove RBCs and clumps.
For NHP immunization studies, blood for serum and PBMC isolation was collected in BD Vacutainer® SST™ tubes and K2 EDTA 5.4 mg tubes, respectively, while animals were safely restrained. Whole blood samples were centrifuged at 3000 RPM for 10 min and sera were collected and stored at −80 °C until testing. Blood for PBMC isolation were retained at RT until processed. Diluted whole blood was layered on 90% Ficoll (GE Healthcare) to isolate PBMCs via density gradient centrifugation at 800 × g. PBMCs were then frozen in Gibco Recovery™ Cell Culture Freezing Medium at 5 × 106 cells/vial and stored in liquid nitrogen until analysis.
Viruses
All viruses used for testing were rescued using a reverse genetics system similar to one previously described54. In brief, an eight-plasmid system was applied for virus rescue. Each bidirectional plasmid encoded one of the eight segmented genes of influenza virus. The sequences for HA and NA genes were strain-specific and the six influenza backbone genes were subtype-specific (IAV or IBV). For IAV, sequences for the backbone genes of PA, PB2, NP, NS, and M were from the A/Puerto Rico/8/1934 (H1N1) (PR8) strain while PB1 sequence was from the A/California/07/2009 (H1N1) strain. For IBV, all six backbone genes were from B/Brisbane/60/2008 (B/Vic). The pool of 2 µg of each plasmid in OptiMEM medium (Gibco # 31985) was co-transfected into a co-culture of HEK-293T and MDCK cells (1:1 ratio) with Lipofectamine 2000 (Invitrogen) for 4 h at 37 °C followed by replacement of media with OptiMEM supplemented with 1 µg/mL of TPCK-treated trypsin. The viruses were harvested at 72-h post-transfection and propagated twice in MDCK cells with multiplicity of infection (MOI) of 0.001–0.01 for passage 1 and MOI of 0.0001–0.001 for passage 2. Passage 1 viruses served as virus seeds and passage 2 viruses served as viral stocks for HAI and MNT assay testing, described below.
Hemagglutination inhibition assay (HAI)
modRNA-HA vaccine-induced functional anti-HA antibodies that prevent HA-mediated agglutination of RBCs were measured using the hemagglutination inhibition assay (HAI). All sera were pre-treated with RDE, heat-inactivated, and then pre-adsorbed with appropriate RBCs to remove any non-specific agglutinins. 2-fold serial dilutions of mouse or NHP sera, tested in duplicate, in PBS were mixed with the vaccine matched influenza virus strain on a shaker for 5 min then left to incubate for 30 min at RT. The neutralization reaction was then mixed with either turkey or guinea pig RBCs (Lampire Biological Laboratories) and incubated an additional 30 or 60 min, respectively, at RT. Assay plates were imaged on a FluHema (SciRobotics). The HAI titer was reported as the reciprocal of the highest serum dilution resulting in loss of HA activity, visualized as a full smear reaching the bottom of the well with substantial footing when the microtiter plate was tilted 60° for 30 s, when using turkey RBCs. If guinea pig RBCs were used, loss of HA activity was observed as a pellet on the microtiter plate without tilting. All samples were run in duplicate.
For the toxicity studies, the HAI assay was conducted by VisMederi (Siena, Italy). In the VisMederi HAI assay, sera collected from mIRV and qIRV immunized rats were pre-treated with RDE, heat-inactivated and then pre-adsorbed with appropriate RBCs to remove any non-specific agglutinins. The treated serum, tested in duplicate per sample, was serially titrated two-fold in a dilution plate starting at a 1:10 dilution. An equal volume of standardized influenza antigen, obtained from Francis Crick Institute (London, UK) and propagated by VisMederi Research (Siena, Italy), was added to the serum samples and the plates were incubated 60 min at RT. RBCs were then added to all wells, and plates were incubated further for 60 min at RT. Following the last incubation, the plates were tilted, and the titer was determined as the reciprocal of the highest serum dilution in which agglutination was still completely inhibited. The geometric mean of four titers per sample (two analysts + two readers) was reported for each influenza vaccine antigen.
Microneutralization test (MNT)
Anti-HA neutralizing antibody responses following vaccination were measured using a 1-day microneutralization assay (MNT). Sera were pre-treated with RDE and heat-activated prior to use in the MNT assay. Serial dilutions of either mouse or NHP sera were incubated in a flat bottom 96-well plate with the vaccine matched influenza virus strain for 1 h at 37 °C/5% CO2. Adherent MDCK cells (MDCK NBL-2, ATCC CCL-34) were added in suspension on top of the neutralization reaction and incubated at 37 °C/5% CO2 for 18–20 h. Cells were then fixed with methanol and stained with either Polyclonal Rabbit IgG Anti-Influenza A NP or Anti-Influenza B NP (Invitrogen) primary antibody followed by AlexaFluor 488 goat anti-rabbit IgG H + L (Life Technologies) secondary antibody. Infected cells were counted using a CTL ImmunoSpot S6 Universal-V Analyzer with ImmunoCapture Software (Cellular Technology Ltd). MNT titers were reported as the reciprocal of the dilution that resulted in 50% reduction in infection when compared to a no serum control. All samples were run in duplicate.
Intracellular cytokine staining assay
Vaccine-induced T cell responses to influenza were measured by flow cytometry-based intracellular cytokine staining assay (ICS). In mouse studies, freshly-isolated splenocytes (2 × 106 cells/well) were cultured in cRPMI with media containing DMSO only (unstimulated) or a specific peptide pool representing HA sequences of the A/Wisconsin/588/2019 (H1N1) influenza virus strain (Mimotopes) for 5 h at 37 °C in the presence of protein transport inhibitors, GolgiPlug and GolgiStop. Following stimulation, cells were stained for surface and intracellular markers to identify activated and/or cytokine-expressing T cell types (CD3+ cells for CD4 vs CD8), activation markers (CD154/CD40L), and cytokines (IFN-γ, IL-2, IL-4, TNF-α, CD154, and CD107a). The eBioscience™ fixable viability dye eFluor 506 (Invitrogen) was used prior to surface staining, per manufacturer’s instructions, to exclude dead cells. After staining, the cells were washed and resuspended in flow cytometry buffer (2% FBS/PBS). Cells were acquired on a BD LSR Fortessa and data were analyzed using BD FlowJo™ software. Results are background (media – DMSO) subtracted and shown as a percentage of CD4+ T cells or CD8+ T cells.
In NHP studies, the ex vivo stimulation with HA peptides was performed as described above, using PBMCs collected at different timepoints in place of splenocytes. Frozen PBMCs were thawed and rested for the ICS assay. Following stimulation, PBMCs were stained for surface and intracellular markers to identify IFN-γ-expressing T cells for both species (rhesus and cynomolgus macaques). Acquired data were analyzed as described above. The T cell gating strategy used for all species is shown in Supplementary Fig. 6A, B.
Toxicology observations and measurements
For in-life assessments, during the Dosing Phase all animals were weighed twice prior to the initiation of dosing (PID) on Days 1 and 7, prior to dosing on Days 1 and 15, and on non-dosing Days 4, 8, and 11, and a fasted weight was collected just prior to scheduled necropsy. Body weights were collected on Recovery Phase Days 1, 8, 15, and 21. Clinical observations occurred at least once daily prior to the initiation of dosing, at least twice daily on non-dosing days and during the recovery phase, and prior to and after each dose on dosing days. Body temperatures were collected on all animals on Dosing Phase Days 1 and 15 prior to dosing and at approximately 4- and 24-h post-dose. Injection sites were observed on Dosing Phase Days 1 and 15 prior to dosing and approximately 4- and 24-h post-dose on all animals.
Hematology was evaluated using a Siemens Advia 2120i analyzer (Siemens Healthineers Tarrytown, NY, USA). Fibrinogen activated partial thromboplastin time, and prothrombin time was evaluated on the Diagnostic Stago STA-R evaluation coagulation analyzer (Diagnostic Stago, Parsippany, NJ, USA). Blood smears were prepared for the first 7 animals on Day 3 and all animals on Day 17 and Day 39. Blood cell morphology was evaluated microscopically on 5 animals of each sex from all groups at both scheduled necropsies (i.e., at dosing and recovery phases). Routine clinical chemistry parameters were evaluated using a Siemens Advia 1800 clinical chemistry analyzer (Siemens Healthineers, Tarrytown, NY, USA). Acute phase proteins alpha-2 macroglobulin (A2M) and alpha-1-acid glycoprotein (A1AGP) were measured using the rat MSD Acute Phase Protein Panel 1 on the MSD SECTOR S 600 Analyzer (Meso Scale Design). Routine urinalysis parameters were measured, and a microscopic examination of sediment for formed elements was performed on 5 animals of each sex from all dose groups at both scheduled necropsies (i.e., dosing and recovery phases).
For post-mortem analysis, complete necropsies, tissue collection, organ weights, and macroscopic tissue evaluation were performed on all animals. Animals were euthanized by gas anesthesia (isoflurane) followed by exsanguination on Dosing Phase Day 17 (2 days after the last dose) or on Recovery Phase Day 22, the last day of the Recovery Phase (surviving animals). Necropsy, tissue collection, organ weights, macroscopic tissue evaluation, and microscopic examination were performed. Anatomic pathology analysis was completed at Pfizer DSRD, Groton, CT, and microscopic examination and peer review were completed at Pfizer DSRD, Pearl River, NY.
Statistical analysis
Animal immunogenicity data was analyzed using GraphPad PRISM software. GMTs of the immune responses for vaccine groups and strains were calculated and are displayed in each bar chart. An independent two sample t-test was performed to compare immune responses of two groups between mIRV or qIRV and QIV (Fluad®). An analysis of variance (ANOVA) was conducted to compare immune responses among mIRV, tIRV, qIRV, and QIV (Fluad®). All pairwise comparisons of the four groups were performed and Tukey’s test was applied to adjust for multiple comparisons. All tests were two-tailed. A p value less than 0.01 was considered statistically significant and is marked with asterisk(s) in the bar charts.
Statistical analysis of body weight, body weight change, food consumption, and body temperature were conducted. Descriptive statistics were generated for each parameter and group at each scheduled sampling time or each time interval. Analysis of body temperature was based on the maximum body temperature post injection for each animal. A nonparametric (rank-transform) one-way ANOVA was conducted, with a 2-sided pairwise comparison of each dose group to the reference group (saline control) using Dunnett’s test. Average ranks were assigned to ties. A p value of less than 0.05 was considered statistically significant and is marked with asterisk(s) in the bar charts.