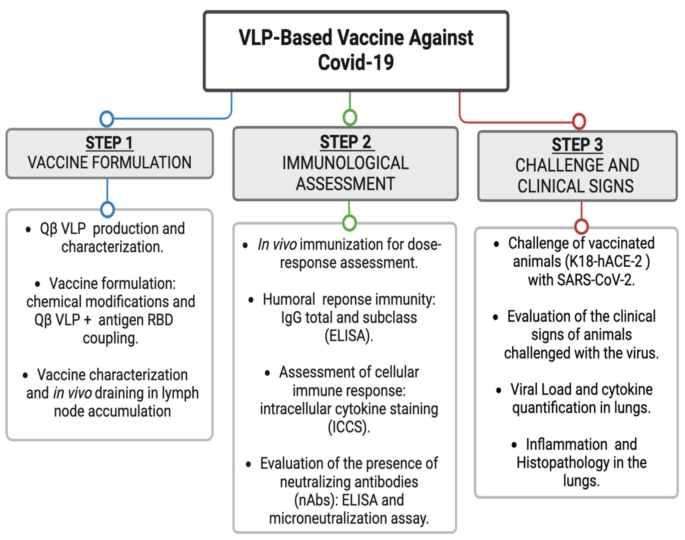
Experimental design
The Covid-19 vaccine investigated in this study was developed through a systematic three-step experimental approach, as outlined in the flowchart (Fig. 1) of the experimental design. Step 1 entailed an understanding of the antigen utilized, specifically the RBD of the Spike protein. We then evaluated the use of VLPs that could be produced efficiently under favorable conditions, characterized by low cost and high immunogenicity. Additionally, the antigen needed to be readily conjugated to the VLPs using a simple and replicable strategy. For this purpose, we selected Qbeta bacteriophage (Qβ) VLPs due to their exposed lysine amino groups, which are well-suited for antigen coupling via bifunctional crosslinkers without disrupting VLPs self-assembly. The vaccine was subsequently formulated and characterized, as detailed in the section “Production and Characterization of the VLPs-Based Vaccine.”
In Step 2, we aimed to determine the optimal vaccine dose and assess the immunological response induced. For this, isogenic C57BL/6 mice were employed to evaluate both the humoral and cellular immune responses elicited by the vaccine formulation, along with the ability of the antibodies to neutralize SARS-CoV-2. The final phase, Step 3, also involved C57BL/6 mice, but specifically those genetically modified to express human ACE2 (human ACE2 knock-in mice). The reasons behind the choice of such an approach are two-fold: to provide consistency of experimental lineage, allowing comparative analysis with previous results, and to adopt a model that expressed the relevant receptor for SARS-CoV-2 infection, facilitating a more accurate assessment of the vaccine’s protective efficacy. Mice were vaccinated with the most effective doses identified from prior experiments. Following vaccination, the mice were challenged with SARS-CoV-2 as described. Clinical signs were monitored, and at the conclusion of the study, viral load and potential tissue damage in the lungs of both vaccinated and unvaccinated challenged mice were evaluated and compared to a control group that was not challenged.
Fig. 1
Flowchart of the experimental design.
Production and characterization of the VLPs-based vaccine
The vaccine was produced using the QβVLPs, based on the coat protein (CP) which forms a uniform VLPs, with an encapsulated toll-like agonist (Fig. 2A and B). For that, synthetic E. coli plasmid of the QβVLPs was constructed by Creative Biostructure (Ramsey Road Shirley, NY 11967, USA), cloning the CP gene from Qβ bacteriophage into pET28a(+) without a 6x-His tag. The constructed plasmid was then used to transform BL21 (DE3) E.coli competent cells obtained from Thermo Fisher Scientific. Recombinant expression of the QβVLPs was carried out in Hannahan’s Broth (SOB medium) for 16 h at 30 °C and 200 rpm. When the optical density at 600 nm (OD600nm) reached 0.6, 1 mM IPTG was added to induce protein expression. After induction, the bacterial cell suspension was centrifuged at 5,000 rpm for 15 min, and the resulting cellular sediment was resuspended in PBS. Subsequently, the cells were lysed by sonication for 15 min using a 35% potency (10 s on and 15 s off). The lysate was then centrifuged, and both the supernatant and pellet fractions were analyzed using SDS-PAGE to assess protein expression. For purification of the QβVLPs, 10% (w/v) PEG 8,000 was added to the supernatant fraction, and the mixture was incubated overnight on a nutating mixer at 4 °C to precipitate total proteins. The precipitate was collected by centrifugation at 9,000 rpm for 30 min. The resulting pellet was resuspended in PBS at pH 7.2 and mixed with an equal volume of a 1:1 chloroform/isobutanol mixture until it formed a colloid. The mixture was then centrifuged at 9,000 rpm for 1 h to separate the layers. The top (aqueous) layer was collected, and dialysis with PBS at pH 7.2 was performed to remove any remaining organic solution.
The total QβVLPs were quantified using the Pierce BCA Protein Assay kit from Thermo Scientific, USA. The purity of the QβVLPs was assessed using SDS-PAGE, Western blotting, and transmission electron microscopy (TEM) (Fig. 2 C-E).
To conjugate the RBD protein to Qβ VLPs, chemical modifications were performed based on established protocols with modifications26,27,28,29. Initially, the RBD protein was incubated with a 7.5-fold excess of SATA (N-succinimidyl-S-acetylthioacetate) for 30 min. Excess SATA was removed by diafiltration, and hydroxylamine hydrochloride was subsequently added at a 1:10 (v/v) ratio. The mixture was incubated at room temperature for three hours to introduce reactive sulfhydryl groups into the RBD protein. Following this, the excess hydroxylamine hydrochloride was removed, and the protein was dialyzed against PES buffer (20 mM sodium phosphate, 2 mM EDTA, and 30% (w/v) sucrose, pH 7.2).
The purified QβVLPs were then treated with SMPH (succinimidyl-6-(b-maleimidopropionamido) hexanoate) cross-linker at a 10-fold molar excess and incubated at room temperature for 30 min. Unreacted SMPH was removed by diafiltration, and the VLPs were dialyzed with PES buffer. Subsequently, the RBD protein, prepared with sulfhydryl groups, was mixed with the SMPH-activated QβVLPs in equimolar amounts. The conjugation reaction was allowed to proceed for four hours at room temperature to achieve covalent linkage of the RBD protein to the QβVLPs.
The vaccine formulation was analyzed using 15% SDS-PAGE under reducing conditions, followed by Coomassie brilliant blue staining. For western blot analysis, the gels were electro blotted onto nitrocellulose membranes (Bio Rad, USA) using Trans-Blot® SD Semi-Dry Transfer Cell Blots (Bio Rad, USA). Besides, aliquots of QβVLPs alone or QβVLPs displaying RBD were used to be analyzed by TEM. For that, the samples were diluted to a concentration of 0.5 µg/µL, and subsequently applied to glow-discharged, carbon-coated Formvar copper grids obtained from Electron Microscopy Sciences30. The dried grids were then examined usin a LEO 906E transmission electron microscope from Zeiss, Germany, operating at an acceleration voltage of 80 kV. Images were acquired at a magnification of 129, 300x.
Stereomicroscopic imaging was performed to analyze the trafficking of the VLPs alone and the vaccine formulation to lymph nodes of mice, as previously described15,30. For that, QβVLPs alone and QβVLPs-RBD were labeled with hyperimmune anti- QβVLPs and anti-RBD and subsequently labeled with Alexa Fluor 488 (AF488) and Alexa Fluor 594 (AF594), according to the manufacturer’s instructions (Thermo Fisher Scientific). The particles were injected subcutaneously (s.c.) into the C57BL/6 mice footpad and measured the draining kinetics. Fluorescent light illumination with a ZEISS Axio Zoom.V16 camera was used for imaging.
Procedures involving animals
All animal experiments in this study were performed in accordance with relevant guidelines and regulations, adhering to Brazilian Federal Law 11.794, which regulates the scientific use of animals, and State Law 11.977, the Animal Protection Code of São Paulo. The project protocol received approval from the Animal Ethics Committee (CEUA) of the Institute of Biomedical Sciences, University of São Paulo (Protocol number 133290720). This study is reported in accordance with ARRIVE guidelines to ensure comprehensive oversight, maximizing research quality, reliability, and transparency.
C57BL/6 mice were purchased from the Central Animal Facility of the Faculty of Medicine at the University of São Paulo, while knockin human ACE2 (B6.Cg-Tg(K18-ACE2)2Prlmn/) mice were obtained from “The Jackson Laboratory,” Bar Harbor, Maine, USA. Animals were housed in ventilated autoclaved cages (Alesco, Brazil) under specific pathogen-free conditions with controlled temperature, humidity, and a 12:12 light-dark cycle. In scientific studies involving the mice, the use of ketamine (100 mg/kg) and xylazine (10 mg/kg) were implemented for anesthesia, offering effective sedation and analgesia. This combination enhances safe handling and reduces stress during experimental procedures. Furthermore, isoflurane served as an inhalational anesthetic for both short and prolonged procedures, ensuring consistent maintenance of anesthesia and stable physiological conditions throughout the study.
At the conclusion of each experiment, animals were humanely euthanized using an approved Schedule 1 method (cervical dislocation). These practices uphold ethical standards and ensure compliance with animal welfare regulations throughout the study.
Assessment of the immunological capacity generated by the vaccine formulation
To assess the capacity of the VLPs-based vaccine to induce humoral and cellular immune response, as well as neutralizing antibodies against the SARS-CoV-2, experiments were conducted using female C57BL/6 mice, and knockin human ACE2 (B6.Cg-Tg(K18-ACE2)2Prlmn/) strains, aged between 6 and 8 weeks (mature immune system).
The vaccination was applied subcutaneously (s.c.) with varying doses of the QβVLPs-RBD vaccine. Initially, we administered sequential doses of the vaccine formulation at 2.5 µg, 5 µg, 10 µg, and 20 µg to assess their efficacy in eliciting an immune response. The doses of 2.5 µg, 5 µg, and 10 µg did not generate a sufficiently robust immunological response. Therefore, we proceeded with the higher doses and included an additional group receiving 50 µg. The 50 µg dose demonstrated a significantly enhanced ability to induce immunity in the murine model. Based on these results, the 20 and 50 µg doses were selected for further investigation due to its superior immunogenicity. As controls, separate groups of mice were immunized with only QβVLPs, RBD, or PBS. The immunizations were administered in two doses, with 14 days of interval between each injection.
To measure the antibody response, blood samples were collected via cheek vein puncture on days 0 (prior vaccination), 7, 14 after the first immunization, and days 7 and 14 after the boost. Following sample collection, all mice were humanely euthanized through CO2 exposure. We performed three independent experiments, with each arm consisting of five female mice, resulting in a total of 15 mice per group.
The production of IgG antibodies, including total and subclass-specific responses, was evaluated using enzyme-linked immunosorbent assays (ELISA), following established protocols with minor modifications26,27,28,29,30. Recombinant Spike protein was diluted to 1 µg/mL in 50 mM carbonate buffer (pH 9.6) and used to coat ELISA plates, which were incubated overnight at 4 °C. The next day, the plates were washed three times with PBS containing 0.05% Tween 20 and subsequently blocked with 1% PBS-BSA for two hours. After blocking, mouse serum samples were added to the plates, starting with a 1:150 dilution, followed by serial three-fold dilutions (1:3) up to four times. The plates were then incubated with goat anti-mouse total IgG HRP conjugate (Sigma-Aldrich/Merck, USA) as the secondary antibody. For subtype identification, goat anti-mouse IgG1 and IgG2b antibodies (Sigma-Aldrich/Merck, USA) were employed. The reaction was developed by adding 100 µL of TMB substrate (Sigma-Aldrich/Merck, USA) to each well and incubating for 10 min at room temperature in the dark. The reaction was halted with 0.5 M H2SO4, and optical density was measured at 450 nm using a microplate reader.
The neutralizing capacity of antibodies was assessed by evaluating the ability of hyperimmune serum from vaccinated mice to inhibit the binding of the RBD to human hACE2. This was performed using the cPass SARS-CoV-2 Neutralization Antibody Detection Kit (GenScript, USA). Serum samples were collected 14 days after the second dose of the QβVLPs-RBD vaccine (administered at 20 µg and 50 µg) and tested alongside controls including QβVLPs, RBD, and PBS. The kit’s provided negative and positive controls were prepared as per the manufacturer’s instructions. For the assay, serum samples were diluted 1:10 and mixed with HRP-conjugated RBD. This mixture was incubated at room temperature for 1 h, then added to a 96-well microplate coated with hACE2. After a 15-minute incubation at room temperature, the microplate was washed with the specific buffer provided by the kit. The reaction was developed by adding the TMB substrate from the kit and was stopped with the provided acidic solution. The absorbance was then measured at 450 nm using a microplate reader.
In addition to utilizing the commercial kit, we conducted an in vitro neutralization assay with SARS-CoV-2 to further evaluate the neutralizing efficacy of serum from vaccinated and control mice. This assay adhered to a well-established and published protocol31, with specific modifications, to ensure the accuracy and reliability of antibody neutralization against the native virus. Serum samples were first heat-inactivated at 56 °C for 1 h to eliminate complement activity. Vero E6 cells, cultured in DMEM supplemented with 5% FBS, were seeded at 104 cells per well in 96-well plates and incubated for 24 h at 37 °C in 5% CO2 to allow for cell adhesion. Serum samples, collected 14 days after the booster dose, were initially diluted 1:20, followed by serial three-fold dilutions (1:3) up to four times. These diluted sera were then incubated with 100 TCID50 of SARS-CoV-2 P.1 (Gamma variant, GenBank: MW642250.1) for 1 h at 37 °C. Following incubation, the virus-serum mixtures were added to the Vero E6 cell plates in DMEM with 1% FBS and further incubated for 72 h at 37 °C in 5% CO2. Negative controls (cells without serum and virus) and positive controls (cells with virus only) were included. After 72 h, the plates were washed and fixed with 4% paraformaldehyde for 20 min. Paraformaldehyde was removed, and the cells were stained with 0.5% crystal violet for 30 min. The excess dye was then washed away with PBS, and the plates were dried before being immersed in methanol. Absorbance was measured at 620 nm using a microplate reader. The experiments were performed in triplicate across three independent assays to ensure reproducibility and reliability of the results.
Cellular immune response was evaluated by intracellular cytokine staining (ICCS). The spleen and lymph nodes of mice were collected two weeks after the boost immunization with VLPs-based vaccine, QβVLPs alone, RBD protein, or PBS. The organs were macerated using a Potter homogenizer. The extracted cells were filtered through a 40 μm cell strainer (Corning, EUA), washed with PBS, and then plated in a 96-well microplate at a concentration of 1 × 106 cells/mL in RPMI-1640 medium supplemented with 10% FBS. For the positive control, cells were incubated with a stimulus solution of PMA + ionomycin in the presence of brefeldin A Stop Golgi (BD Bioscience, USA). The negative control cells were treated with PBS + brefeldin A Stop Golgi. To detect cytokines, T cells from the immunized mice were incubated with 5 µg of RBD + brefeldin A Stop Golgi®. All samples were then incubated for 12 h at 37 °C in a 5% CO2 environment. After incubation, th cells were fixed and permeabilized using the Cytofix/Cytoperm™ Plus Fixation/Permeabilization kit (BD Biosciences, USA). They were then stained with anti-mouse CD4+ (BD Bioscience, USA), anti-mouse CD8+ (BD Bioscience, USA), and anti-mouse IFNγ (BD Bioscience, USA) antibodies. Cytometry analysis was performed using the FACS Canto II flow cytometer (BD Biosciences, EUA), and the data were analyzed using FlwJo software (Flowjo, EUA, https://www.flowjo.com/solutions/flowjo) and GraphPad Prism 9.1 (Graphpad Software, EUA https://www.graphpad.com/).
Measuring vaccine protective capacity and safety
Following evaluation of immune induction capacity of the vaccine formulation, we measured protection against viral infection and safety. Female B6.Cg-Tg(K18-ACE2)2Prlmn mice were vaccinated and challenged with P1. SARS-CoV-2. Prior to vaccination, the B6.Cg-Tg(K18-ACE2)2Prlmn mice were genotyped according to The Jackson Laboratory recommendations. Each group consisting of five animals were vaccinated as described previously. On the 35th day following the immunization schedule, the animals were intranasally challenged with 106 virus particles of P.1 SARS-CoV-2 diluted in 40 µL of PBS. Clinical signs, behavior, and weight loss were monitored for seven days.
Following the observation period, the right lung of each animal was collected and preserved in 10% formalin solution buffered with phosphate for a duration of seven days. The lung specimens were then embedded in paraffin and processed using a tissue processor (PT05 TS, LUPETEC, UK). Subsequently, histological paraffin (Histosec, Sigma-Aldrich) was used to embed the lung samples. Thin sections measuring 4 μm in thickness were prepared and stained with hematoxylin and eosin. The resulting sections were captured using a Slide Digitizer − 3D Histech scanner and subsequently analyzed using Download CaseViewer 2.4, 64-bit version (3D- Histotech, Hungary) – https://www.3dhistech.com/solutions/caseviewer/). Finally, the figures were compiled using Adobe Photoshop version 23.5.4 (USA, https://www.adobe.com/br/products/photoshop.html).
Viral quantification was measured by qRT-PCR. Total RNA was extracted from homogenized mice lungs using Trizol Reagent. To synthesize cDNA, the Superscript II Reverse Transcriptase (Thermo Fisher) was employed, in accordance with the manufacturer’s instructions. Real-time PCR was conducted using the Power SyBr green master mix (Thermo Fisher) and a QuantStudio 12k thermocycler (Thermo Fisher), utilizing the following parameters: an initial denaturation step at 95 °C for 15 min, followed by 40 cycles of denaturation at 95 °C for 15 s and annealing/extension at 60 °C for 1 min. The primer sequences used were as follows: CoV-2 forward 5′- GCCTCTTCTCGTTCCTCATCAC-3′ and CoV-2 reverse 5’- AGCAGCATCACCGCCATTG − 3’. The qRT-PCR data were presented as 1/ΔCT and were performed in triplicate.
In addition, we also measured the cytokine expression profile in the lung tissue of vaccinated and control animals by RT-qPCR. The assay was performed as described above for viral load quantification. The oligonucleotides used are shown in Table 1.
Table 1 Oligonucleotides sequences used to quantify cytokine mRNAs by RT-qPCR.
Statistical analysis
The statistical analysis of all data was conducted using GraphPad Prism 9.1 for Mac (GraphPad Inc, USA, https://www.graphpad.com/). For comparisons between two normally distributed groups, an unpaired t-test was utilized, taking into account the distribution of the data. Alternatively, when comparing two non-parametric groups, a Mann–Whitney rank test was applied. In cases involving more than two groups, non-parametric data were assessed using one-way ANOVA, with Bonferroni’s multiple comparison post-test applied to normally distributed data. Weight measurements and flow cytometry data were subjected to analysis via Two-Way ANOVA. Survival analysis was performed using the log-rank test. Statistical significance was determined at P ≤ 0.05.