Baseline characteristics of participants in the phase 2 and booster trials are summarized in Table 1, stratified by age and sex. The incidence of all solicited AEs in these studies has been previously reported5,7. Additionally, tables showing the incidence of AEs stratified by severity, gender, and age are provided in Supplementary 1 (Tables S1, S2, and S3).
Table 1 Demographics and baseline characteristics of participants in phase 2 and booster trials.
Effect of age and gender on seroconversion and reactogenicity
Analysis of the effects of age and gender on the incidence of seroconversion and AEs using a log-binomial regression model is shown in Table 2.
Table 2 Log-binomial regression analysis of age and gender and induced local and systemic adverse events with seroconversion.
There was a consistent trend for slightly lower seroconversion rates in men than in women after the first, second, and third doses, but the difference after the first and third doses was not significant. I didn’t reach it. Therefore, a significant effect of age on seroconversion was only seen after the second dose (RR 0.99, p = 0.048).
The incidence of injection site pain after the first and booster doses was significantly influenced by age (RR 0.99, p < 0.001 and RR 0.99, p = 0.019, respectively). Furthermore, after the booster dose, men had a significantly lower risk of injection site swelling/induration than women (RR 0.6, p = 0.031).
After the first dose, gender-adjusted age had a significant effect on the reported rates of fatigue, headache, and myalgia (Table 2). After the first dose, older age was associated with a small but significant decrease in the rates of fatigue (RR 0.97, p < 0.001), headache (RR 0.98, p = 0.034) and myalgia (RR 0.97, p = 0.016). was. After the second dose, only fatigue rate was affected by age (RR 0.98, p = 0.017). After the third booster dose, no effect of age on AEs was evident.
After the first dose, there was an age-adjusted gender effect on AEs, with males having a lower incidence of fatigue (RR 0.81, p = 0.046), headache (RR 0.64, p = 0.002), and myalgia (0.72, p) was significantly lower. = 0.043) than women. After the second dose, men’s rates of fatigue (RR 0.78, p = 0.07), headache (RR 0.71, p = 0.052), and myalgia (0.68, p = 0.054) tended to decrease again, but these The difference is borderline important. The same trend of decreased AEs in men was seen after the third booster dose, with lower AEs for fatigue (RR 0.7, p = 0.074), headache (RR 0.49, p = 0.003), and myalgia (0.65, p = 0.154). The percentage has decreased. However, only the difference in headache was statistically significant.
Data on seroconversion rates stratified by sex and age are provided in Figure S1 in Supplementary 1.
Relationship between age, gender and serum neutralizing antibody concentration
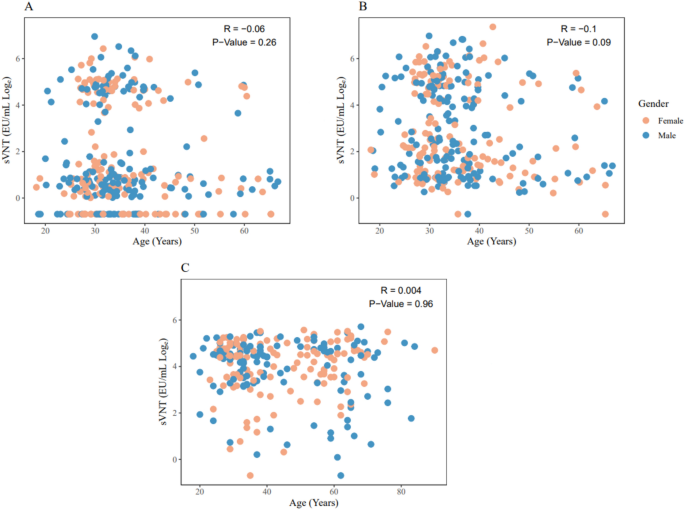
There was no significant correlation between age and surrogate virus neutralization test (sVNT) results after the first, second, or third vaccination (Figure 1).
Figure 1
Correlation of sVNT responses and age as a function of gender status. Scatterplots of the sVNT response and age distribution after the first and second injections (A and B) and the sVNT response and age distribution after the booster study injection (C) are shown.
Relationship between the incidence of local and systemic adverse events and neutralizing antibody levels
We then analyzed the relationship between age-adjusted serum neutralizing antibody levels and the incidence of injection site pain and swelling, fatigue, headache, myalgia, and arthralgia (Table 3). Age-adjusted neutralizing antibody levels after the first, second, or third dose showed no correlation with AEs (Table 3).
Table 3. Relationship between post-vaccination adverse events and age-adjusted neutralizing antibody levels.
Source link