Study design
The study protocol has been disclosed in detail (15). The trial was a single-blind, single-center randomized controlled trial and the study was conducted from March 2023 to March 2024. The study was conducted using quantitative methods of research and the effect assessment protocol used clinical reagent tests as well as internationally recognized scales. This study was conducted at the Fourth Hospital of Harbin Medical University, Harbin, Heilongjiang Province, China, and was approved by the Ethics Committee of the Fourth Hospital of Harbin Medical University (2022-WZYSLLSC-20). Written informed consent was obtained from all participants. Registration (ChiCTR2200064321) was completed with the China Clinical Trial Registry (www.chictr.org.cn) before the start of the trial. This research report complies with the CONSORT-eHEALTH reporting standard (see Additional file 1, Complete CONSORT-eHEALTH Form V 1.6.1 for details).
Study randomization
Given that the patient population was socially dispersed DTC patients and that patients coming to the hospital were far from meeting the trial needs of the minimum study sample size at one time in the short term, we included patients by consecutive enrollment into the group. The researchers recruited patients with a combination of inclusion and exclusion criteria as well as patients who demonstrated a positive attitude and commitment to their treatment plan when they came to the hospital to assess their potentially higher adherence to future treatment. Patients who agreed to participate in the study were randomized to either the MTM-mHealth group or the control group, followed by a baseline assessment. Given that the total number of patients registered at the hospital visit on a daily basis is uncertain, we generated random numbers by means of the function algorithm RANDBETWEEN in the computer software EXCEL to ensure that randomized allocation was fully achieved. For a detailed randomization strategy, please see the disclosure of the previous study protocol (15).
Study participants
We began recruiting patients in March 2023 and ended recruiting patients in March 2024, with patient recruitment occurring after radical surgery and prior to the start of iodine-131 therapy and endocrine therapy. Patients will be required to undergo a minimum of 2 weeks after undergoing radical surgery to ensure that they have recovered from the procedure to a state where randomization assignments can be made. Of note is that according to treatment guidelines (16), after a period of recovery following the completion of radical surgery for thyroid cancer, patients come to the hospital for a review of TSH levels ≥ 30.0 mU/L before iodine-131 therapy can be administered. Each patient was enrolled in the trial and signed an informed consent form at the hospital after a face-to-face meeting with the attending physician. (Please see Additional file 4 for informed consent.) Participants in this study were recruited on a consecutive enrollment basis, so not all patients started the intervention at the same time. However, all participants completed the 3-month intervention cycle. Inclusion and exclusion criteria were as follows:
Inclusion criteria:
(1)
Patients were ≥ 18 years old;
(2)
Patients were resident in Heilongjiang province (annual time away from home less than 1 month);
(3)
The patients all met the relevant diagnostic criteria for thyroid cancer in the Guidelines for the Diagnosis and Treatment of Thyroid Nodules and Differentiated Thyroid Cancer, and were diagnosed with differentiated thyroid cancer by pathological examination;
(4)
Patients were clinically diagnosed with a high risk of DTC recurrence risk stratification;
(5)
Radiation iodine-131 therapy and endocrine therapy were given after receiving radical surgery for thyroid cancer;
(6)
The patients use smartphones on a daily basis and are familiar with the general function of WeChat;
(7)
Patients voluntarily participated in the study and signed an informed consent form.
Exclusion criteria:
(1)
The patient has no fixed contact information, no family member is responsible for contact, and it is not convenient to contact by phone or WeChat;
(2)
Patients taking any psychotropic drugs;
(3)
The patients had psychiatric symptoms such as delirium, slurred speech, and uncooperative;
(4)
Patients with a combination of any of the following serious systemic diseases: heart failure (NYHA class III or IV); cirrhosis of the liver in Child–Pugh class B or C; end-stage renal disease requiring dialysis or peritoneal dialysis; severe chronic obstructive pulmonary disease (COPD) with FEV1 < 30% of predicted; organ dysfunction or failure due to endocrine disorders, and severe somatic functional impairment and participation in other clinical trials;
(5)
Pregnant or lactating women; and
(6)
The patient is not expected to complete the required follow-up or treatment cycles within the next year.
Sample size
The study sample size was estimated based on the primary outcome (change in anxiety and depression levels) using G*Power, version 3.1.9.7 (University of Dusseldorf) (17). To the best of our knowledge, similar intervention studies using the PHQ-4 to assess similar outcomes do not yet exist, and therefore effect sizes for the primary outcome of anxiety and depression levels, respectively, were considered. The pooled effect sizes for anxiety and depression in previous studies were 0.63 and 0.59, respectively, with 80% efficacy at a 5% two-sided significance level (18). The sample size required for studies of changes in anxiety levels was approximately 18, while for studies of changes in depression levels, the sample size required was approximately 21. Based on the estimation results, in order to saturate the study sample size, the sample size for this two-group parallel trial would need to be at least 21 participants per group to detect an effect size of at least 0.50 on the post-intervention change score for the primary outcome, which, taking into account a potential attrition rate of 20%, would require a sample size of 54 patients, with 27 patients in each group.
Treatment programs
Two groups of patients received I-131 treatment and TST therapy (19, 20). Before treatment, thyroid uptake scans were performed to detect whether there was residual or recurrent thyroid cancer (21). Patients took 50–200 mCi iodine-131 according to individual conditions and were observed in the isolation ward for 3 days to reduce the risk of radiation. On the second day of treatment, levothyroxine sodium tablets were taken orally. The initial dose was usually 50 μg per day, the maximum was not more than 100 μg, and the maintenance dose was 50–200 μg per day. Four weeks later, the thyroid function was reviewed and the dose was adjusted according to the results. During the subsequent maintenance phase (22), the goal of treatment was to maintain TSH levels in the range of 0.3–0.5 mU/L to optimize therapeutic efficacy and reduce adverse effects.
Interventions
This study established a multidisciplinary team composed of a thyroid cancer pharmacist, a nursing expert, a thyroid cancer doctor, and a public health expert. After several rounds of discussion, the team developed a comprehensive intervention strategy. In the control group, patients received standard medical and nursing services, while the intervention group received additional MTM-based mHealth intervention on this basis.
The personalized intervention program for the intervention group included:
Online health education: Through an intelligent WeChat platform, patients in the intervention group were able to access immediate health education resources. The platform not only supports direct communication between doctors and patients so that patients can get professional answers quickly, but also allows doctors or nurses to provide real-time interventions to patients, such as reminding the time to take medication, and providing popularization of thyroid cancer-related knowledge (please see the multimedia database for materials used in popularization of the science, please refer to the Additional file 2).
Comprehensive medical care: From the first day of hospitalization, patients in the intervention group received comprehensive medical care services including WeChat group health education, online health lectures, personalized health education programs, and drug supervision. During the outpatient treatment, the patients continued to receive professional guidance, and after discharge, through mobile health education services, for a period of 3 months of intervention.
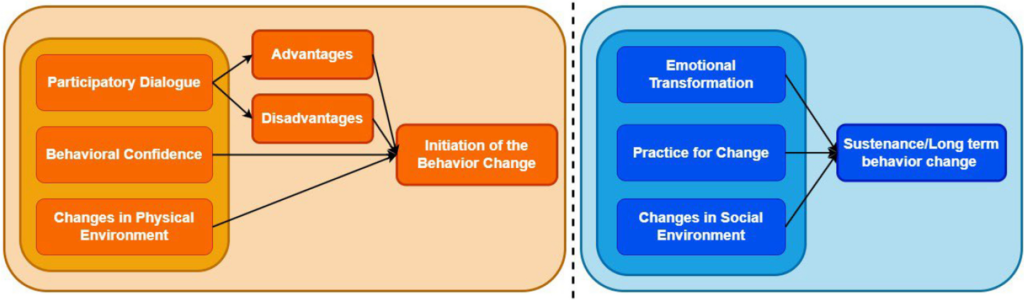
MTM-based intervention content design: The entire intervention process follows the MTM theory to ensure that the content is scientific and relevant.
1.
Participatory dialogue: Physicians distribute educational brochures to encourage patients to improve their health behaviors, and regular communication through WeChat emphasizes positive facing of the disease.
2.
Behavioral confidence: Through health behavior diaries and patient communication, patients’ confidence in changing health behaviors is enhanced.
3.
Changes in physical environment: Provide online educational resources to guide patients to change their daily diet and living habits.
4.
Emotional change: guiding patients to focus on health behavior change, reminding them of precautions through WeChat, helping them make long-term plans, and taking different measures depending on their compliance.
5.
Practical change: reflecting and adjusting health behaviors through patients’ sharing diaries and exchange salons.
6.
Social environment change: motivate patients to change health behaviors through WeChat communication and psychological support, and encourage or guide patients in time.
The control group received a standard program of care to ensure that they received the basics of thyroid cancer treatment and lifestyle guidance. This included a detailed Health Education for Thyroid Cancer Patients booklet, which covered the basics of thyroid cancer, sensible dietary advice, guidance on medication, and information on possible adverse effects.
At the time of discharge, patients are informed of the anticipated follow-up schedule so that they understand the importance of and expectations for follow-up visits. At the end of the first month after discharge, the first telephone follow-up was carried out to ensure a smooth transition from the patient to the home treatment environment. Each month thereafter, regular follow-up phone calls are made to monitor the patient’s health status, treatment compliance, and any potential problems. These follow-up calls provide an opportunity for patients to ask questions, share their experiences, and receive support and guidance from the care team. Through this routine pattern of follow-up visits, patients in the control group were able to maintain contact with their healthcare professionals and receive the necessary help when they encountered difficulties in their daily lives.
The specific flow of the study is shown in Fig. 2, which clearly demonstrates the stages and key aspects of the study, and the complete and detailed intervention program has been disclosed (15).
Fig. 2
Outcome measures
This study used Questionstar, a web-based survey instrument, to collect study questionnaires completed by all participants at baseline and follow-up.
Clinical pharmacists were responsible for distributing the questionnaires through Questionnaire Star and providing the necessary standardized instructions to patients during the completion process, while clinical pharmacists were unaware of the grouping of patients. For hospitalized patients, the pharmacist’s guidance was provided through face-to-face communication; for post-discharge patients, guidance was provided through telephone communication to ensure that all patients were able to complete the questionnaire successfully. Patient demographic information was collected through the case system and supplemented with self-designed questionnaires to obtain more comprehensive and accurate data. The questionnaire design used in this study followed strict scientific standards to ensure the accuracy and reliability of the data collection process.
Primary outcome
Anxiety and depression levels
In this study, symptoms of depression and anxiety were assessed using a simplified version of the Anxiety Depression Scale (PHQ-4). The PHQ-4 scale is a validated brief self-report scale suitable for assessing the frequency of depressive and anxiety symptoms in an individual over the past 2 weeks. The PHQ-4 scale has been designed to be concise, straightforward, easy to administer, and easy to understand, allowing healthcare professionals to quickly screen individuals with symptoms of anxiety and depressive disorders, to and provide an initial assessment. The scale consists of two main dimensions: depression and anxiety, and contains a total of four entries. The first two entries are designed to assess depressed mood, while the last two are used to assess anxiety (23). Each entry was rated on a four-point scale, i.e., “not at all,” “a few days,” “more than half the days,” and “almost every day,” corresponding to scores of 0, 1, 2, and 3, respectively. The total score of the scale ranges from 0 to 12. In a study in China, the Cronbach’s alpha coefficient of the PHQ-4 scale was 0.833, indicating high internal consistency reliability and validity (24). Its Cronbach’s alpha coefficient in this test was 0.839.
Secondary outcomes
Fear of cancer recurrence
In this study, the Fear of Cancer Recurrence Scale (FCR-4) was used to assess the patients’ level of concern about cancer recurrence (25). The FCR-4 scale is based on the simplification of the original “FCR-7” scale, which was developed by Simard and Savard in 2018 and has already shown good reliability in the previous study, with a Cronbach’s alpha coefficient of 0.86 (26). The use of the FCR-4 scale helps healthcare professionals to accurately identify patients’ fears of cancer recurrence and provide customized mental health support and resources accordingly. The scale consists of four questions designed to provide insight into cancer patients’ fear of cancer recurrence. The questions are rated on a five-point Likert scale ranging from “never” to “always” to quantify the patient’s level of fear. The Cronbach’s alpha coefficient was 0.885 in this trial.
Quality of life
The quality of life was assessed using the EQ-5D-5L scale, which is an updated and improved version of the EQ-5D scale. It adds five different levels based on the five dimensions of the former to provide a more detailed description of health status (27, 28). These five dimensions are Mobility, Self-care, Usual activities, Pain/Discomfort, and Anxiety/Depression, each of which is subdivided into No Difficulty, Little Difficulty, Moderate Difficulty, Great Difficulty, and Complete Difficulty. Difficulty, Major Difficulty, and Complete Difficulty. The EQ-5D-5L has been shown to have high validity and reliability in seven studies of cancer patients with health utility values ranging from 0.62 to 0.90 (29,30,31,32,33,34,35).
Compared to the traditional EQ-5D (EQ-5D-3L), the EQ-5D-5L retains the same dimensional and hierarchical structure, but the number of ratings per dimension has been increased from three to five, which improves the granularity of the assessment. In addition, the EQ-5D-5L includes a visual analog scale (VAS) that allows individuals to rate their current state of health on a scale of 0 to 100 based on how they feel. This score provides a continuous variable to measure an individual’s overall quality of life. Its Cronbach’s alpha coefficient in this trial was 0.678.
Satisfaction with health education
Patient satisfaction was assessed by the customer satisfaction questionnaire (CSQ-3), and the questionnaire was adapted to better adapt to the purpose of this study. The CSQ-3 is a widely used instrument designed to assess customer satisfaction with a product, service, or experience, and has demonstrated good reliability and validity in a number of domains, with a Cronbach’s alpha coefficient of 0.84 (36, 37). The questionnaire was developed by Roger, D et al. in 1993 and has been widely used in market research, customer relationship management, and corporate decision-making (38, 39). Its Cronbach’s alpha coefficient in this trial was 0.837.
Statistical analysis
The study was statistically analyzed using SPSS Statistics 27.0 software. Count data were presented as frequencies and percentages (%). For continuous data, normality tests were performed to determine the distributional characteristics of the data. Data were described as mean ± standard deviation (x̄ ± s) if they conformed to normal distribution, and median and interquartile range (IQR) if they did not. We conducted a series of comparisons to assess specific hypotheses for within- and between-group differences in outcome variables at different time points between the two groups. We used the Mann–Whitney U test to compare the change in scores for each scale between the two groups at baseline and endpoint. And for the differences in the scale scores at baseline and endpoint within the two groups, we used the Wilcoxon test for comparison. Since the data did not satisfy the assumption of normal distribution, non-parametric tests were considered appropriate. The effect size r (Pearson’s correlation coefficient) was calculated by dividing the Z value by the square root of the total sample size to quantify the mean difference between the two groups. In addition, the demographic characteristics between the two groups were compared using the chi-square test. All statistical tests were two-sided, and P < 0.05 was considered statistically significant.