ethics
This study was approved by the Medical Ethics Committee of the Chinese Academy of Medical Sciences and Peking Union Medical University (Beijing, China) (CAMS&PUMC-IEC-2022-019, March 14, 2022) and was conducted in accordance with the Declaration of Helsinki. The need for informed consent was waived by the ethics committee because the study utilized de-identified hospitalization data collected as part of routine clinical practice. This exemption was granted on the basis that the study posed minimal risk to participants and that the retrospective nature of the study made obtaining consent impractical.
data source
This study used influenza vaccination records in Beijing from January 1, 2016 to December 31, 2018, obtained from the Beijing Center for Disease Control and Prevention. In addition, electronic medical records for hospitalizations in Beijing from 2012 to 2020 were linked from the Beijing Health Big Data and Policy Research Center, a city-wide hospitalization surveillance system in Beijing, China 16,17. Due to incomplete vaccination data for residents from other provinces, study subjects were limited to permanent residents of Beijing (identified based on basic medical insurance information in hospitalization data).
research design
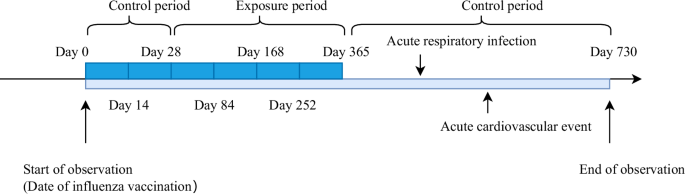
A self-controlled case series study (SCCS) was conducted. The exposure factor was influenza vaccination and the outcome of interest was acute cardiovascular events. The time of influenza vaccination was considered as the start of the observation period, and the time of hospitalization for acute cardiovascular events was considered as the time of disease onset. The observation period was defined as from the date of influenza vaccination (day 0) to 730 days thereafter. Because protective antibodies from influenza vaccines are typically generated 2 to 4 weeks after vaccination,18,19 and influenza vaccines are typically administered once a year, this study defined the exposure period as: From day 29 after vaccination to 365 days thereafter. The control period was defined as the observation period excluding the exposure period. That is, 0 to 28 days and 366 to 730 days after influenza vaccination. The method of dividing this time interval is similar to that used in a previous study20.
According to previous studies, acute respiratory infections are a risk factor for acute cardiovascular events21,22. Therefore, this study also considered acute respiratory infections occurring after vaccine administration and before the outcome event as a confounding exposure factor with an exposure window set from 0 to 3654 days post-infection. A schematic diagram of the observation period is shown in Figure 1.
Figure 1: Observation period of the self-administered case series study.
A 2-year observation period was set, with the first year after influenza vaccination as the exposure period and the second year after influenza vaccination as the control period.
To minimize potential confounding factors, such as the impact of COVID-19 vaccines, this study focused on analyzing hospital records dated before December 31, 2020. As a result, influenza vaccination implementation was restricted to dates before December 31, 2018. The self-controlled case series analysis ensures a full 2-year observation period for each individual. Considering that influenza vaccination is typically received annually, it was assumed that the protective efficacy of influenza vaccine would decline to baseline levels in the second year if influenza vaccination was not received during the 2-year observation period. Inclusion criteria are as follows: (1) Received an influenza vaccination between January 1, 2016 and December 31, 2018, and has not received a subsequent influenza vaccination within two years. (2) occurrence of at least one acute cardiovascular event within 2 years after influenza vaccination; Exclusion criteria were as follows: (1) If an individual has multiple vaccination records that meet the above conditions, the first record will be retained. (2) Exclusion of Individuals Under 18 Years of Age. The inclusion and exclusion flowchart is shown in Figure 2.
Figure 2: Flowchart of participant inclusion and exclusion from the study.
Participants were individuals who were vaccinated between January 1, 2016 and December 31, 2018 and who had experienced at least one acute cardiovascular event within 2 years.
For patients who experienced multiple acute cardiovascular events during the observation period, due to the potential association between these events, this study used the time point of the first acute cardiovascular event during the observation period as the time point of analysis. I considered it.
Exposure and consequences
The exposure factor was influenza vaccination. China has licensed trivalent (IIV3) and quadrivalent inactivated influenza vaccines (IIV4), including split-virus, whole-virus, and subunit vaccines. The specific types of vaccines utilized during the study season are shown in Supplementary Table 1. All licensed influenza vaccines contain strains recommended for the Northern Hemisphere by the World Health Organization (https://www.who.int/teams/global-influenza-). Programs/vaccines/who-recommendations; http://www.chinacdc.cn/en/). Supplementary Table 2 shows the influenza vaccine strains, prevalent strains, predominant circulating strains, and concordance between vaccine and circulating strains for the 2015–2016 to 2018–2019 influenza seasons.
The outcome event was an acute cardiovascular event, which was identified by International Classification of Diseases, 10th Revision (ICD-10) hospital discharge diagnosis code. Acute cardiovascular events include acute myocardial infarction, stroke, acute myocarditis, acute pericarditis, and other acute cardiovascular diseases. Chronic or subacute diseases such as cerebral infarction (I63.903) and chronic ischemic heart disease (I25) are not included. Detailed disease codes are provided in Supplementary Table 3.
Regarding influenza vaccination status, if the patient’s influenza vaccination records are inconsistent, it indicates that the patient has not received influenza vaccination or received the vaccination outside of Beijing City. The study was limited to patients with medical insurance in Beijing, so it is unlikely that people will receive the vaccine outside of Beijing. Therefore, in this study, we consider patients without matching influenza vaccination records to have not received influenza vaccination.
Other factors
Acute respiratory infections were considered as another exposure factor because of their complex association with acute cardiovascular events21,22. This study could not identify the causative agent of the acute respiratory infection because laboratory data were not available. Therefore, the scope of acute respiratory tract infections in this study includes not only influenza infections, but also acute upper respiratory tract infections (ICD-10: J00-J06) and lower respiratory tract infections (ICD-10: J09-J18, J20). ~J22) is also included. , these were identified by International Classification of Diseases, 10th Revision (ICD-10) hospital discharge diagnosis codes. The study also took seasonal factors into account, including spring (March 21st to June 21st), summer (June 22nd to September 22nd), autumn (September 23rd to December 21st), We were divided into four groups for winter (from December 22nd). March 20th of the following year). Medical history information for the study population was obtained from previous hospital records, specifically all hospitalization records from 2012 until the onset of the first acute cardiovascular event during the observation period. Medical history information includes cardiovascular disease (ischemic heart disease and stroke), hypertension, hyperlipidemia, chronic lower respiratory disease, ulcers, diabetes, kidney disease, liver disease, and cancer. ICD-10 diagnosis codes for these diseases can be found in Supplementary Table 3.
statistical methods
Basic characteristics of participants were described according to type of acute cardiovascular event. Continuous and categorical variables were described using medians (quartiles) and frequencies (percentages), respectively. Wilcoxon rank sum test and chi-square test were used to compare differences between groups. Additionally, this study also characterized and compared patients with different cardiovascular disease histories.
In this study, we performed an analysis of overall acute cardiovascular events and their subtypes (e.g., myocardial infarction, ischemic stroke, hemorrhagic stroke). Analyzes of acute cardiovascular disease subtypes were based on patients with a discharge diagnosis that included only that type of acute cardiovascular disease. Patients with multiple types of events occurring simultaneously were excluded. If a patient was hospitalized with an acute respiratory infection multiple times within a 45-day period, the multiple hospitalizations were considered one episode of acute respiratory infection, and the first hospitalization was considered the time of disease onset. Ta. The same data processing method was applied to acute cardiovascular events.
Relative incidence of acute cardiovascular events during the exposure period (29–365 days post-vaccination) compared to baseline using a standard SCCS model and adjusting for acute respiratory infection factors and seasonal effects (RI) and 95% confidence interval (CI) were calculated. (days 0–28, 366–730 after vaccination) and at different times within the exposure period (days 29–84, 85–168, 169–252, 253–365). R.I. This study also investigated the protective effect of influenza vaccination against acute cardiovascular events within 1 year and its variation over time in individuals with various histories of cardiovascular disease.
In this study, we conducted a stratified analysis of the preventive efficacy of influenza vaccines using age, gender, underlying disease, and occurrence of acute respiratory infections as stratification factors. Likelihood ratio tests were used to investigate potential interactions between influenza vaccine administration and these factors. Additionally, to investigate the impact of multiple inactivated influenza vaccine (IIV) vaccinations on the prevention of acute cardiovascular events, we grouped individuals based on whether they had received another influenza vaccination within the year before the observation period. Classified. A person was classified as having received multiple vaccinations if they had received another influenza vaccination within 1 year before the current vaccination. Otherwise, it is classified as having received one vaccination. As only data on influenza vaccination records from September 1, 2015 onwards were available, the earliest start of the observation period was set 1 year later, specifically September 1, 2016.
To ensure the robustness of the results, the following sensitivity analysis was performed. (1) Not adjusted for acute respiratory infections. (2) Build separate models for different influenza seasons. (3) exclude patients who experienced both an acute respiratory infection and an acute cardiovascular event during the same hospitalization; (4) Excludes patients who died due to acute cardiovascular events during hospitalization. Additionally, we extended the observation period to 3 years to investigate the effects of IIV immunization beyond 1 year. The exposure period was defined as 29 to 730 days after vaccination, and the control period was defined as 0 to 28 days and 731 to 1095 days after vaccination. Only individuals who received an influenza vaccination between January 1, 2016 and December 31, 2017 and who had not received a subsequent vaccination within 3 years were included in the analysis.
Data analysis was performed using SAS software (version 9.4), R software (version 4.2.3), and RStudio (version 2023.12.0). The SCCS model was built using the “SCCS” package in R. All statistical tests were two-sided, and the significance level was set at 0.05.