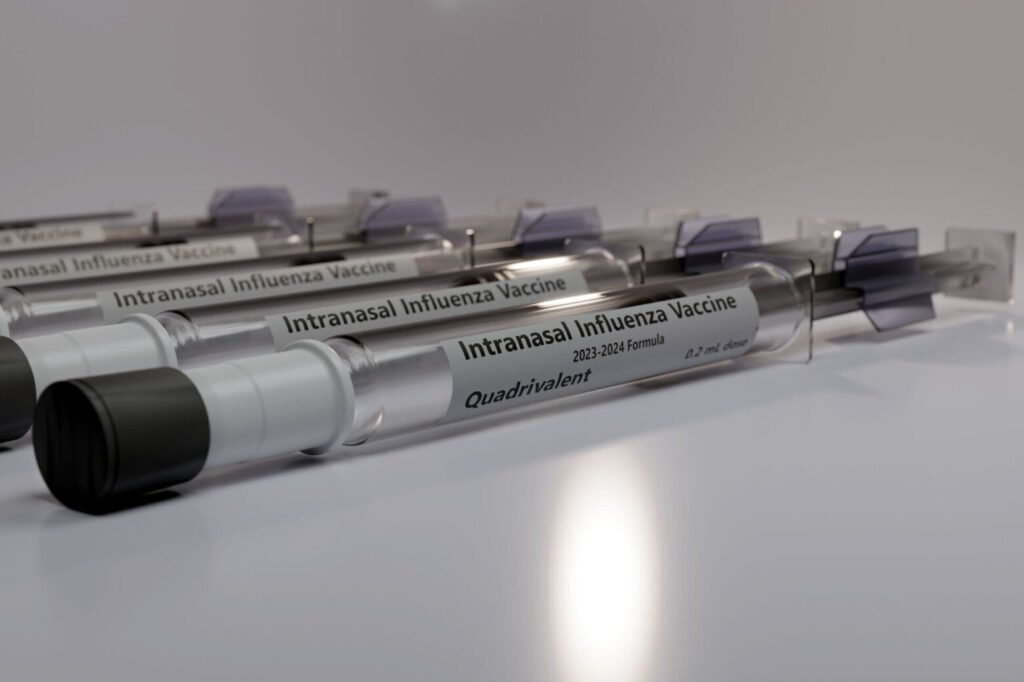
Gaithersburg, Md.-based MedImmune has received FDA approval for its FluMist nasal spray influenza vaccine, which means no doctor is needed.
Gaithersburg, Maryland-based MedImmune has received Food and Drug Administration approval for its FluMist nasal spray influenza vaccine for self-administration or caregiver administration. That means you don’t need a doctor.
It will be available at online pharmacies starting next year.
FluMist still requires a prescription, but is approved for home administration to individuals ages 2 to 49 years. People administering the vaccine to themselves or others must be 18 years of age or older and must complete a screening and eligibility assessment. Once approved, the vaccine will be shipped to the address provided during the order.
For children between the ages of 2 and 17, the drug must be administered by an adult guardian, the FDA said.
“The approval of the first influenza vaccine for self- or caregiver administration could increase convenience, flexibility, and accessibility for individuals and families to receive a safe and effective seasonal influenza vaccine.” “We offer new options for customers,” said Director Peter Marks. FDA’s Center for Biologics Evaluation and Research.
MedImmune was acquired by AstraZeneca in 2007 for $15.6 billion.
FluMist was originally approved in 2003 as an alternative to traditional influenza vaccination. It is sprayed directly into the nose.
FluMist is a live attenuated influenza vaccine. That is, it contains a weakened version of the influenza virus.
The Centers for Disease Control and Prevention estimates that from 2010 to 2023, influenza caused 9.3 million to 41 million illnesses per year, as many as 710,000 hospitalizations, and 51,000 deaths per year. .
Sign up here to get the latest news and daily headlines delivered to your email inbox.
© 2024 WTOP. All rights reserved. This website is not directed to users within the European Economic Area.