Overall analysis
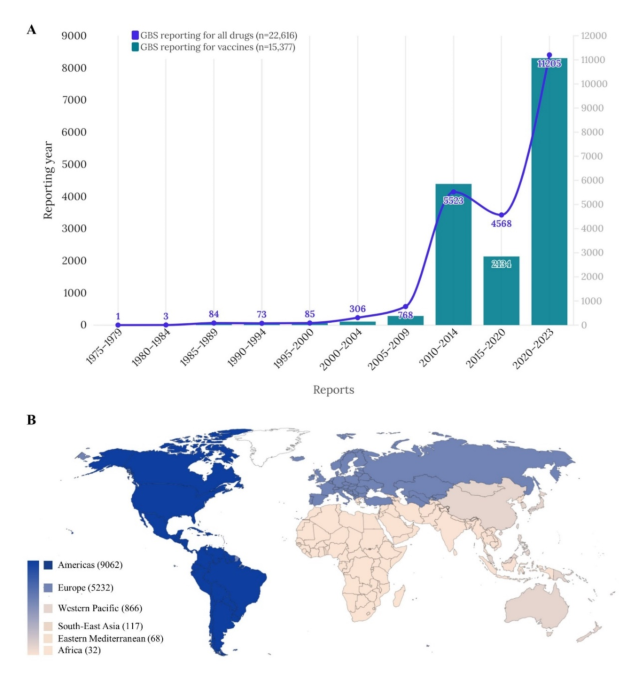
Imbalance analysis on a dataset containing 15,377 cases of vaccine-related GBS (8,072 men (52.49%)) recorded in VigiBase between 1978 and 2023, out of 8,010,602 reports in the complete database. was conducted (Table 1). We categorized the reported incidents into six geographic regions, as shown in Figure 1. More than half (58.93%) of the reports came from the Americas region, followed by Europe (34.02%) and the Western Pacific region (5.63%). %). The majority of reports were related to COVID-19 mRNA vaccines (29.17%), followed by influenza vaccines (26.25%), and Ad5 vectored COVID-19 vaccines (16.23%). Ta. Reports of GBS were distributed in the following age groups: 0-11 years (6.43%), 12-17 years (5.22%), 18-44 years (23.26%), 45-64 years (28.74%), and 65 years. I was there. or more (23.68%). The mean TTO was 5.47 days with a standard deviation of 41.72 (Table 1). A sub-analysis of vaccine-associated GBS based solely on reports from medical professionals is shown in Table 2. TTOs for individual vaccines are shown in Table 3.
Table 1 Baseline characteristics of reports of vaccine-associated GBS adverse events in the WHO pharmacovigilance database, VigiBase, from 1967 to 2023 (n = 15,377). 1
Temporal trends (A) and global distribution (B) of vaccine-associated GBS adverse events by continent (total n = 15,377). GBS Guillain-Barré syndrome.
Table 2 Sub-analysis of disproportional occurrence of vaccine-related GBS adverse events Table 3 Detailed report (heat map) for each vaccine related to GBS and concomitant adverse events.
Vaccine-related GBS disparity analysis
Most vaccines, except rotavirus and tuberculosis vaccines, showed significant associations with GBS (Table 4). Influenza vaccine had the highest association with GBS (ROR, 77.91 (95% CI, 75.30 to 80.62); IC, 5.98 (IC025, 5.93)), followed by typhoid vaccine (ROR, 42.52 (95% CI) , 35.59-50.80), IC025, 5.93). IC, 5.17 (IC025, 4.87)), hepatitis A vaccine (ROR, 32.67 (95% CI, 29.39-36.32); IC, 4.94 (IC025, 4.76)), rabies vaccine (ROR, 28.24 (95% CI, 22.36) -) 35.67); IC, 4.56 (IC025, 4.17)), yellow fever vaccine (ROR, 24.67 (95% CI, 20.6-29.56); IC, 4.48 (IC025, 4.17)), papillomavirus vaccine (ROR, 17.60 ( 95% CI), 15.96–19.41), IC, 4.08 (IC025, 3.92)), hepatitis B vaccine (ROR, 15.99 (95% CI, 14.27–17.92), IC, 3.94 (IC025, 3.75)), ad5 vector New coronavirus infection vaccine (ROR, 14.88 (95% CI, 14.26-15.53); IC, 3.66 (IC025, 3.59)), meningococcal vaccine (ROR, 13.90 (95% CI, 12.51-15.43); IC, 3.75 (IC025, 3.57)), pneumococcal vaccine (ROR, 10.47 (95% CI, 9.57 to 11.46); IC, 3.34 (IC025, 3.19)), COVID-19 mRNA vaccine (ROR, 9.66 (95) % CI, 9.33-10.00); IC, 2.84 (IC025), 2.80)), varicella-zoster vaccine (ROR, 9.62 (95% CI, 8.67-10.68); IC, 3.23 (IC025, 3.05)), DTaP-IPV -Hib vaccine (ROR, 9.31 (95% CI, 8.8 to 9.86); IC, 3.14 (IC025, 3.05)), MMR vaccine (ROR, 7.03 (95% CI, 6.24 to 7.92); IC, 2.78 (IC025, 2.58)), inactivated whole virus coronavirus vaccine (ROR, 3.29 (95% CI, 2.65-4.09), IC, 1.69 (IC025, 1.33)), and encephalitis vaccine (ROR, 2.26 (95% CI, 1.91- 2.66); IC, 1.16 (IC025, 0.89)).
Table 4 Analysis of the disproportionate incidence of vaccine-related GBS adverse events.
When we examined the correlation between GBS and total vaccine doses across different age groups, a significant association was evident in all age groups. The significance of this correlation was observed to increase with age. The highest association was found in people aged 65 years and older (IC, 4.19 (IC025, 4.13)), followed by the age group 45-64 years (IC, 3.67 (IC025, 3.62)) and 18-44 years (IC, 2.93 (IC025, 2.87)), 12-17 years (IC, 2.86 (IC025, 2.75)), 0-11 years (IC, 1.92 (IC025, 1.81)). When individual vaccines were analyzed, influenza, varicella-zoster, COVID-19 mRNA, and ad5-vectored COVID-19 vaccines showed higher associations with older age groups. Inactivated whole-virus COVID-19 vaccines showed the highest association exclusively in the 12- to 17-year-old age group. Other vaccines showed the highest association in the 18-64 age group. For example, rabies vaccine (IC, 4.32 (IC025, 3.76)), yellow fever vaccine (IC, 4.69 (IC025, 4.12)), DTaP-IPV-Hib vaccine (IC, 5.45 (IC025, 5.25)), meningococcal Vaccine ( IC, 5.63 (IC025, 5.36)), Pneumococcal vaccine (IC, 4.36 (IC025, 4.05)), Typhoid vaccine (IC, 5.12 (IC025, 4.57)), Encephalitis vaccine (IC, 5.31 (IC025, 4.82) ), hepatitis A vaccine (IC, 5.57 (IC025, 5.31)), hepatitis B vaccine (IC, 4.30 (IC025, 4.01)), MMR vaccine (IC, 4.28 (IC025, 3.91)), and papillomavirus vaccine (IC , 4.10 (IC025, IC025, 3.75)).
Most routinely administered vaccines tend to show a stronger association with increasing age, with the exception of the papillomavirus vaccine, which is recommended starting at age 11 or 12. I am. For papillomavirus vaccines, the highest level of association is observed in the 18-44 age group. Among non-routine vaccines, COVID-19 vaccines tend to show a strong association with increasing age, likely because booster shots are given to older age groups. It is thought that. Other nonroutine vaccines, such as rabies, yellow fever, typhoid, encephalitis, and tuberculosis vaccines, tend to have the highest association in the 18- to 64-year age group. This is likely due to higher social participation and travel activities across these age groups.
Examining differences based on gender, males (ROR, 31.68 (95% CI, 30.48-32.93), IC, 3.43 (IC025, 3.39)) and females (ROR, 33.92 (95% CI, 32.51-35.39), IC, 3.45 (IC025, 3.41)). Both men (IC, 4.25 (IC025, 4.18)) and women (IC, 4.15 (IC025, 4.06)) showed the highest association with the 65+ age group, with stronger associations with increasing age. Observed. A detailed description of the reports regarding vaccine-associated GBS is provided in Table 3.
Cumulative report analysis
The cumulative number of vaccine-related GBS reports is shown in Figure 2. Only a few reports were documented before 2010, but the overall number of reports has since increased dramatically with the emergence of reports related to multiple vaccines. Furthermore, from mid-2020, the number of reports rapidly increased with the introduction of vaccines related to the new coronavirus infection, and the highest proportion was reported for the new coronavirus infection mRNA vaccine, followed by the Ad5 vector. This was followed by a new coronavirus vaccine.
Figure 2
Cumulative number of annual reports of GBS adverse events associated with different vaccines (A-C). GBS, Guillain-Barre syndrome.