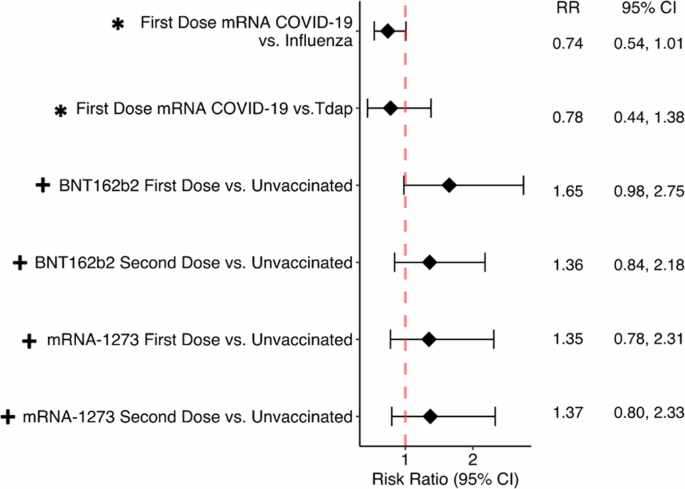
Dorney et al1 found that mRNA COVID-19 vaccination did not increase the risk of retinal vein/artery occlusion (RVO), but Li et al2 found that all COVID-19 vaccines increased the risk of RVO. I discovered that. Although our studies may appear to yield different conclusions, we aim to show where and why our studies agree. Our analysis showed that mRNA vaccination 21 days after first COVID-19 vaccination from 2020 to 2022 compared with patients who received influenza and Tdap vaccination from 2018 to 2019. We specifically evaluated the risk of developing RVO1. We stratified by brand of COVID-19 vaccine and looked at the risk of developing RVO compared to unvaccinated patients both 12 weeks and 2 years after vaccination. At 12 weeks, neither study showed an association between COVID-19 vaccination and RVO (Figure 1). Previous literature on RVO after COVID-19 vaccination reports onset within days to up to 6 weeks after vaccination, with clinically relevant timelines ranging from months to years. Instead, it is on a scale of days to weeks1,3. This is further supported by a recent review of ocular adverse events after vaccination, where the mean time from vaccination to adverse event was 10.8 days.
Figure 1: Forest plot assessing the risk of retinal vessel occlusion 3 and 12 weeks after mRNA COVD-19 vaccination.
This forest plot compares the risk of retinal vessel occlusion at 3 and 12 weeks after mRNA COVID-19 vaccination. *Dorney et al. Study 1 (assessed 3 weeks after COVID-19 vaccination). + Li Study 2 (assessed 12 weeks after COVID-19 vaccination).
Li’s study included restrictive exclusion criteria that excluded patients taking anticoagulants, antiplatelet drugs, contraceptives, diuretics, and other commonly prescribed medications compared to our study. Additionally there was some selection bias and uncontrolled confounders. Given that many American patients take at least one of these classes of drugs, the generalizability of this study is greatly limited. Furthermore, when comparing vaccinated and non-vaccinated individuals, these two groups may have fundamentally different access to care and health behaviors, and the rate of RVO reporting in the unvaccinated control group may be artificially reduced. Concerns arise as there is the potential to minimize the
While we applaud their attempt to exclude COVID-19 positive patients and include only COVID-19 negative patients, there are several flaws in the methodology used. The study excluded 562,700 patients with confirmed COVID-19 infection from the 7.3 million patients surveyed, which could mean significant underreporting of COVID-19 cases. is high. This analysis excludes only documented positive COVID-19 tests at TriNetX affiliated facilities, including the proportion of asymptomatic patients, patients being tested at home, or other health care providers. Tests are missing. The CDC reported that 146.6 million Americans, or 43.9% of the population, may have been infected with the coronavirus6. Therefore, approximately 3.2 million patients in this cohort may have had COVID-19 infection but were still included. Coronavirus disease (COVID-19) is a highly thrombotic disease7 with a known risk of RVO1,2. Therefore, by including only one patient with a negative COVID-19 test, it is likely that 43.9% of patients had COVID-19 at some point over a two-year period. is insufficient to accurately understand. This will bias both the vaccinated patient group and the unvaccinated patient group, especially those who did not have protection from the vaccine, from contracting COVID-19.
Additionally, patients were required to have a negative COVID-19 test to participate in the study, but this negative result could change at any time throughout the two-year study period. Therefore, it is likely that many patients included in the analysis contracted COVID-19 at some point within 2 years, which is in contrast to the 2 years post-vaccination and COVID-19 infection reported by Li et al. The increased risk associated with the disease vaccine may also have had an impact. ‘s study. Our analysis had the advantage of using 2018 and 2019 influenza and Tdap vaccinations as controls, eliminating COVID-19 infection as a potential confounder in the control group. Finally, this inclusion criterion also means that patients who test negative for COVID-19 will only be tested if they have known exposure or symptoms similar to COVID-19. I’ve overlooked it. This inclusion criterion therefore significantly reduces the patient pool from 95 million patients to just 6.7 million, resulting in the inclusion of only 739,066 vaccinated individuals and the known The study also failed to include people who tested negative for the coronavirus and had no exposure or symptoms.