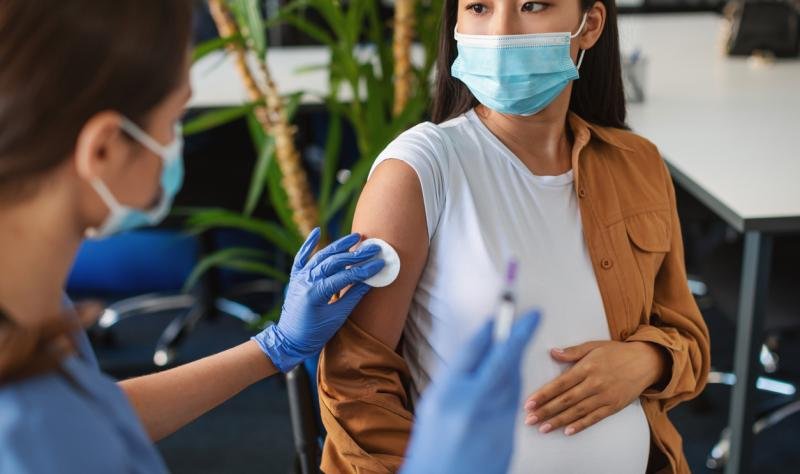
Last year, for the first time in the United States, two methods were approved to combat respiratory syncytial virus (RSV) infections in infants. One is a maternal vaccine given during the last week of pregnancy, and the other is the antibody nisevimab, given to infants under 8 months of age during RSV. season.
However, in the Morbidity and Mortality Weekly Report, the authors show that only 55.8% of infants were protected by maternal RSV vaccine, nisevimab, or both. It describes the survey results.
To evaluate the use of maternal vaccines and infant antibodies, CDC will survey women ages 18 to 49 who reported pregnancy at any time after August 1, 2023, and by March 26, 2024. A web-based survey was conducted from April 11th.
The study included 866 women with infants born between August 2023 and March 2024. Analysis of RSV vaccination coverage was limited to 678 women who were 32 to 36 weeks pregnant at any point between September 1, 2023, and January 31, 2024.
We evaluated nilsevimab coverage in infants and the proportion of infants protected by maternal or infant vaccination in 866 women who gave birth between August 1, 2023 and March 31, 2024.
Provider recommendations greatly influence adoption
Maternal RSV vaccination coverage was 32.6%, of which 54.1% reported receiving the vaccine at their obstetrics and gynecology clinic. The authors said several factors were associated with maternal vaccine use. The participation rate was higher among those with private or military insurance (38.9%) than with public insurance (28.0%). Those living above poverty (35.0%) and those living below poverty (26.4%). The rate was higher for those with a college degree or higher (50.1%) than for those with a college degree or less (28.7% to 32.7%).
Of note, only 1.9% of women who did not receive a doctor’s recommendation received the vaccine, compared with 56.7% of women who relied on a recommendation from their health care provider.
“About half of pregnant women report not receiving health care provider recommendations for maternal RSV vaccination or infant nilsevimab, representing a missed opportunity to protect infants from severe RSV disease. ”, the authors write.
The rate of infant nilsevimab treatment was 44.6%. Again, uptake was higher among those who received a health care provider’s recommendation for maternal or infant RSV vaccination (58.7%) compared with those who did not receive a recommendation (28.3%).
Overall, 55.8% of infants were protected by maternal RSV vaccination, nisevimab, or both. 14.2% of infants were protected by both. These numbers were below the predicted percentages.
Coverage may have been lower than expected due to multiple challenges in rolling out new immunization products.
“Multiple challenges in rolling out new immunization products may have resulted in lower coverage than expected,” the authors wrote. “These challenges included timing of recommendations and product availability, differences in recommendations regarding the timing of vaccine and nisevimab administration, and concerns about product safety and efficacy.”
Antiviral treatments show promising results
In a related RSV study, promising results from a phase 3 randomized, placebo-controlled trial conducted in China showed that gilesovir, a novel antiviral treatment for RSV, significantly lowered viral loads at day 3 and clinical bronchiolitis. It has been shown to significantly reduce scores.
The study, published in the New England Journal of Medicine, included 244 children and infants hospitalized with RSV.
No safety concerns were noted.
“These initial findings warrant further evaluation of gilesovir treatment for RSV infection in an international phase 3 trial,” the authors concluded.